Atypical Teratoid Rhabdoid Tumors Market
Key Highlights
- Adults’ atypical teratoid/rhabdoid tumor is a challenge in terms of diagnosis and management due to several factors, such as its rarity, which accounts for the paucity of data about management and outcome, its complex and wide histologic spectrum, and its aggressive natural history associated with worse prognosis.
- There are currently no FDA-approved therapies specifically for atypical teratoid/rhabdoid tumor, and treatment relies on conventional multimodal approaches including surgery, chemotherapy, and radiotherapy.
- Pipeline research emphasizes molecularly guided therapies, aiming to exploit the unique genetic and epigenetic vulnerabilities of SMARCB1/SMARCA4-deficient tumors for more durable responses.
- Several investigational drugs are in development for atypical teratoid/rhabdoid tumor, including Nivolumab, Alisertib, ONC206, Tazemetostat, and other early-phase agents targeting immune checkpoints, mitotic regulation, and epigenetic vulnerabilities.
- High recurrence rates, aggressive progression, and limited treatment options highlight the urgent need for safe, effective, and disease-specific therapies, supported by dedicated clinical trials and translational research.
DelveInsight’s "Atypical Teratoid/Rhabdoid Tumor – Market Insight, Epidemiology, and Market Forecast – 2034" report delivers an in-depth understanding of atypical teratoid/rhabdoid tumor, historical and forecasted epidemiology as well as the atypical teratoid/rhabdoid tumor market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The atypical teratoid/rhabdoid tumor market report provides current treatment practices, emerging drugs, atypical teratoid/rhabdoid tumor share of individual therapies, and current and forecasted atypical teratoid/rhabdoid tumor market size from 2020 to 2034, segmented by seven major markets. The report also covers current atypical teratoid/rhabdoid tumor treatment practices/algorithms and unmet medical needs to curate the best of the opportunities and assess the underlying potential of the market.
Geography Covered
- The United States
- EU4 (Germany, France, Italy, and Spain) and the United Kingdom
- Japan
Study Period: 2020–2034
|
Study Period |
2020–2034 |
|
Forecast Period |
2025–2034 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain) and the UK, and Japan |
|
Atypical Teratoid/Rhabdoid Tumor |
Segmented by
|
|
Atypical Teratoid/Rhabdoid Tumor Companies |
|
|
Atypical Teratoid/Rhabdoid Tumor Therapies |
|
|
Atypical Teratoid/Rhabdoid Tumor Market |
Segmented by
|
|
Analysis |
|
Atypical Teratoid/Rhabdoid Tumor Disease Understanding and Treatment Algorithm
Atypical Teratoid/Rhabdoid Tumor Overview
Atypical teratoid/rhabdoid tumor is a rare and highly aggressive central nervous system (CNS) tumor that primarily occurs in young children. It is defined by the loss of the SMARCB1 (INI1) gene, or less commonly SMARCA4, leading to dysfunction of the SWI/SNF chromatin-remodeling complex and widespread epigenetic dysregulation. Clinically, AT/RT often presents with symptoms such as headaches, vomiting, seizures, or focal neurological deficits, depending on the tumor’s location within the CNS. Diagnosis typically involves neuroimaging to identify characteristic mass lesions, histopathological evaluation revealing rhabdoid cells, immunohistochemistry showing loss of INI1 expression, and molecular testing to confirm the underlying genetic alterations. Prognosis remains poor due to the tumor’s aggressive nature and high recurrence rates, with limited availability of targeted treatment options and significant challenges in clinical management.
Atypical Teratoid Rhabdoid Tumor Diagnosis
The diagnosis of atypical teratoid/rhabdoid tumor typically involves a combination of imaging, pathology, and molecular testing. Magnetic Resonance Imaging (MRI) is the primary imaging modality, often revealing a large, heterogeneous intracranial mass with areas of necrosis, hemorrhage, and variable enhancement patterns depending on the tumor location. Histopathological examination of the tumor tissue shows rhabdoid cells characterized by eccentric nuclei, prominent nucleoli, and eosinophilic cytoplasm. Immunohistochemistry is crucial, with loss of INI1 (SMARCB1) or BRG1 (SMARCA4) nuclear expression serving as a diagnostic hallmark. Molecular genetic testing confirms underlying alterations in the SMARCB1 or SMARCA4 genes, helping to differentiate AT/RT from other embryonal brain tumors.
Further details related to diagnosis will be provided in the report…
Atypical Teratoid Rhabdoid Tumor Treatment
Treatment of atypical teratoid/rhabdoid tumor typically follows a multimodal approach aimed at aggressive tumor control. The mainstay includes maximal safe surgical resection to remove as much of the tumor as possible, followed by intensive multi-agent chemotherapy using drugs such as vincristine, cyclophosphamide, etoposide, cisplatin, and methotrexate. Radiotherapy is usually incorporated, although its use in very young children is often delayed or modified due to concerns about long-term neurocognitive toxicity. In addition to conventional approaches, targeted and experimental therapies are under investigation. The EZH2 inhibitor tazemetostat has shown promise in early-phase trials, particularly in tumors with SMARCB1 loss, while immunotherapy and gene therapy approaches are being explored in clinical studies. Despite these efforts, prognosis remains poor, and treatment strategies continue to evolve as new molecular targets are identified.
Further details related to treatment will be provided in the report…
Atypical Teratoid/Rhabdoid Tumor Epidemiology
The atypical teratoid/rhabdoid tumor epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by the total incident cases of atypical teratoid/rhabdoid tumor, gender-specific cases of atypical teratoid/rhabdoid tumor, age-specific cases of atypical teratoid/rhabdoid tumor and total treatable cases of atypical teratoid/rhabdoid tumor in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
- As per the secondary search, the overall incidence was 0.084 per 100,000 population in the US.
- AT/RT represented 18.3% of all CNS malignant tumors diagnosed in infants aged < 1 year, with a relatively high incidence of 0.583.
- The age-adjusted incidence was reported to be 0.07 per 100,000 population under 19 years in the US.
- Notably, the incidences in young children aged 1–4 years, children aged 5–9 years and young adolescents were significantly lower than those in infants younger than 1 year.
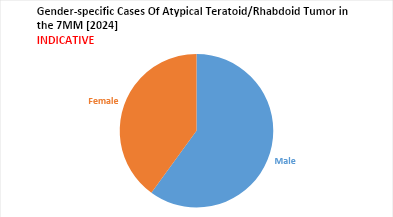
- The male predominance was accounted for more than 60% of all patients.
Atypical Teratoid/Rhabdoid Tumor Recent Developments
- In September 2025, Lantern Pharma announced the successful completion of a Type C meeting with the FDA, receiving key guidance on the regulatory pathway and trial design for its planned pediatric CNS cancer trial, including Atypical Teratoid Rhabdoid Tumor (ATRT).
Atypical Teratoid/Rhabdoid Tumor Drug Chapters
Key emerging players in the atypical teratoid/rhabdoid tumor space include Ono Pharmaceuticals and Bristol Myers Squibb (Nivolumab), Jazz Pharmaceuticals (ONC206), Takeda Pharmaceuticals (Alisertib), among others. The drug chapter also helps understand the atypical teratoid/rhabdoid tumor clinical trial details, expressive pharmacological action, agreements and collaborations, approval, and patent details, and the latest news and press releases.
Emerging Drugs
Nivolumab: Ono Pharmaceuticals and Bristol Myers Squibb
The company had initiated the Phase II trial in japan for the treatment of patients who are refractory or intolerant to one or more regimens of chemotherapy for rhabdoid Tumor.
ONC206: Jazz Pharmaceuticals
ONC206 is a dual targeted investigational therapy that is an agonist of the mitochondrial protease ClpP and an antagonist of the G protein-coupled receptor DRD2. The Company is conducting Phase I dose escalation studies of ONC206 to evaluate safety and pharmacokinetic data in recurrent and rare primary central nervous system neoplasms including teratoid rhabdoid tumor.
In April 2025, Jazz Pharmaceuticals announced the successful completion of its acquisition of Chimerix, for approximately USD 935 million in cash. Chimerix is now a wholly owned subsidiary of Jazz.
|
Comparison of Emerging Drugs Under Development | ||||
|
Drug Name |
Company |
Highest Phase |
Indication |
MoA |
|
Nivolumab |
Ono Pharmaceuticals and Bristol Myers Squibb |
II |
Rhabdoid tumor |
PD-1 inhibitor |
|
Alisertib |
Takeda Pharmaceuticals |
II |
Recurrent or Progressive CNS atypical teratoid/rhabdoid tumors |
Aurora A kinase inhibitor |
|
ONC206 |
Jazz Pharmaceuticals |
I |
Recurrent and rare primary CNS neoplasms including Teratoid/rhabdoid tumor |
ClpP agonist and DRD2 antagonist |
Note: Detailed emerging therapies assessment will be provided in the final report.
Drug Class Insight
The drug classes currently in development for the treatment of atypical teratoid/rhabdoid tumor encompass a wide range of mechanisms, including PD-1 inhibitor (Nivolumab), Aurora A kinase inhibitor (Alisertib), C ClpP agonist and DRD2 antagonist (ONC206), among others.
PD-1 inhibitor
In atypical teratoid/rhabdoid tumor, tumor cells can express PD-L1, which binds to the PD-1 receptor on T cells and suppresses their activity, allowing the tumor to evade immune detection. PD-1 inhibitors, such Nivolumab, block this interaction, preventing the inhibitory signal and keeping T cells active. This enables T cells to recognize and attack the tumor cells, enhancing the immune response against AT/RT.
Atypical Teratoid/Rhabdoid Tumor Market Outlook
The treatment landscape for atypical teratoid/rhabdoid tumor remains heavily reliant on conventional multimodal approaches, including maximal surgical resection, intensive chemotherapy, and radiotherapy, with no therapies currently approved specifically for atypical teratoid/rhabdoid tumor. Off-label use of agents such as chemotherapeutics (vincristine, cyclophosphamide, etoposide, cisplatin, methotrexate), immune checkpoint inhibitors (e.g., OPDIVO), and targeted kinase inhibitors (e.g., alisertib) is common, guided by pediatric oncology protocols and limited clinical evidence. Despite aggressive treatment, outcomes are poor, particularly in very young children, underscoring the urgent need for novel, disease-specific therapies.
Emerging research is increasingly focused on targeted and mechanism-based strategies. Nivolumab is being investigated for its ability to reactivate T cells against tumor cells that evade immune surveillance. Alisertib, a selective Aurora A kinase inhibitor, disrupts mitotic pathways, inducing cell cycle arrest and apoptosis in rapidly dividing AT/RT cells. ONC206, an agonist of the mitochondrial protease ClpP and an antagonist of the DRD2 receptor, triggers mitochondrial dysfunction and apoptosis, representing a novel therapeutic approach.
Other investigational agents target epigenetic vulnerabilities, immunomodulatory pathways, and oncogenic signaling specific to SMARCB1- or SMARCA4-deficient tumors. These efforts reflect a shift toward precision medicine approaches tailored to the molecular and immunological features of AT/RT. Given the absence of FDA-approved therapies, treatment decisions rely on off-label use and expert consensus, emphasizing the critical need for dedicated clinical trials and development of safe, effective, disease-specific therapies.
Further details will be provided in the report….
Atypical Teratoid/Rhabdoid Tumor Drugs Uptake
This section focuses on the uptake rate of potential drugs expected to be launched in the market during 2025–2034. The landscape of atypical teratoid/rhabdoid tumor treatment has experienced a profound transformation with the uptake of novel medicines. These innovative therapies are redefining standards of care.
Atypical Teratoid/Rhabdoid Tumor Pipeline Development Activities
The report provides insights into different therapeutic candidates in Phase II, and Phase I stage. It also analyzes key players involved in developing targeted therapeutics.
Pipeline Development Activities
The report covers detailed information on collaborations, acquisitions and mergers, licensing, and patent details for atypical teratoid/rhabdoid tumor emerging therapies.
KOL- Views
To keep up with current market trends, we take KOLs and SMEs' opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Some of the leaders like MD, Professor and Vice Chair of the Department of Oncology and Director, PhD, and others. Their opinion helps to understand and validate current and emerging therapies and treatment patterns or atypical teratoid/rhabdoid tumor market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Delveinsight’s analysts connected with 30+ KOLs to gather insights; however, interviews were conducted with 10+ KOLs in the 7MM. Centers such as the Washington University School of Medicine, University Medical Center Hamburg-Eppendorf, and University Graduate School of Medicine etc. were contacted. Their opinion helps understand and validate atypical teratoid/rhabdoid tumor epidemiology and market trends.
|
KOL Views |
|
“The high frequency of metastasis at diagnosis and poor survival outcome highlighted the malignant biological behavior of atypical teratoid/rhabdoid tumor. A traditionally aggressive multimodal regimen is necessary to improve the prognosis; this regimen should include surgical resection, particularly maximal resection, combined with chemotherapy and radiotherapy. In the future, based on molecular analysis, the genetic therapeutic model should be a promising regimen for atypical teratoid/rhabdoid tumor.” |
Qualitative Analysis
We perform qualitative and market intelligence analysis using various approaches, such as SWOT and conjoint analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
The analyst analyzes multiple emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry.
In efficacy, the trial’s primary and secondary outcome measures are evaluated.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials.
Market Access and Reimbursement
Reimbursement may be referred to as the negotiation of a price between a manufacturer and a payer that allows the manufacturer access to the market. It is provided to reduce the high costs and make the essential drugs affordable. Health technology assessment (HTA) plays an important role in reimbursement decision-making and recommending the use of a drug. These recommendations vary widely throughout the seven major markets, even for the same drug. In the US healthcare system, both Public and Private health insurance coverage are included. Also, Medicare and Medicaid are the largest government-funded programs in the US. The major healthcare programs, including Medicare, Medicaid, Health Insurance Program (CHIP), and the state and federal health insurance marketplaces, are overseen by the Centers for Medicare & Medicaid Services (CMS). Other than these, Pharmacy Benefit Managers (PBMs) and third-party organizations that provide services and educational programs to aid patients are also present.
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of currently used therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Further detailed analysis will be provided in the report….
Scope of the Report
- The report covers a descriptive overview of atypical teratoid/rhabdoid tumor, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into atypical teratoid/rhabdoid tumor epidemiology and treatment.
- Additionally, an all-inclusive account of both the current and emerging therapies for atypical teratoid/rhabdoid tumor is provided, along with the assessment of new therapies, which will have an impact on the current treatment landscape.
- A detailed review of the atypical teratoid/rhabdoid tumor market; historical and forecasted is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies, by understanding trends shaping and driving the 7MM atypical teratoid/rhabdoid tumor market.
Atypical Teratoid Rhabdoid Tumor Report Insights
Atypical Teratoid Rhabdoid Tumor Report Insights
- Patient Population
- Therapeutic Approaches
- Atypical Teratoid/Rhabdoid Tumor Pipeline Analysis
- Atypical Teratoid/Rhabdoid Tumor Market Size and Trends
- Market Opportunities
- Impact of Upcoming Therapies
Atypical Teratoid Rhabdoid Tumor Report Key Strengths
- Ten Years Forecast
- 7MM Coverage
- Atypical Teratoid/Rhabdoid Tumor Epidemiology Segmentation
- Key Cross Competition
- Highly Analyzed Market
- Drugs Uptake
Atypical Teratoid Rhabdoid Tumor Report Assessment
- Current Treatment Practices
- Unmet Needs
- Analyst Views
- Pipeline Product Profiles
- Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
FAQs
- What was the atypical teratoid/rhabdoid tumor market share (%) distribution in 2024 and what it would look like in 2034?
- What would be the atypical teratoid/rhabdoid tumor total market size as well as market size by therapies across the 7MM during the study period (2020–2034)?
- What are the key findings about the market across the 7MM and which country will have the largest atypical teratoid/rhabdoid tumor market size during the study period (2020–2034)?
- At what CAGR, the atypical teratoid/rhabdoid tumor market is expected to grow at the 7MM level during the study period (2020–2034)?
- What would be the atypical teratoid/rhabdoid tumor market growth till 2034 and what will be the resultant market size in the year 2034?
- What are the disease risks, burdens, and unmet needs of atypical teratoid/rhabdoid tumor?
- What is the historical atypical teratoid/rhabdoid tumor patient pool in the United States, EU4 (Germany, France, Italy, and Spain), and the UK, and Japan?
- What would be the forecasted patient pool of atypical teratoid/rhabdoid tumor at the 7MM level?
- What will be the growth opportunities across the 7MM concerning the patient population of atypical teratoid/rhabdoid tumor?
- Among the 7MM which country would have the most incident cases of atypical teratoid/rhabdoid tumor during the study period (2020–2034)?
- At what CAGR the population is expected to grow across the 7MM during the study period (2020–2034)?
- How many companies are developing therapies for the treatment of atypical teratoid/rhabdoid tumor?
- How many emerging therapies are in the mid-stage and late stage of development for the treatment of atypical teratoid/rhabdoid tumor?
- What are the key collaborations (industry–industry, industry-academia), mergers and acquisitions, and licensing activities related to atypical teratoid/rhabdoid tumor therapies?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- What are the clinical studies going on for atypical teratoid/rhabdoid tumor and their status?
- What are the key designations that have been granted for the emerging therapies for atypical teratoid/rhabdoid tumor?
- What are the 7MM historical and forecasted market of atypical teratoid/rhabdoid tumor?
Reasons to buy
- The report will help in developing business strategies by understanding trends shaping and driving the atypical teratoid/rhabdoid tumor market.
- To understand the future market competition in the atypical teratoid/rhabdoid tumor market and insightful review of the SWOT analysis of atypical teratoid/rhabdoid tumor.
- Organize sales and marketing efforts by identifying the best opportunities for atypical teratoid/rhabdoid tumor in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identification of strong upcoming players in the market will help in devising strategies that will help in getting ahead of competitors.
- Organize sales and marketing efforts by identifying the best opportunities for the atypical teratoid/rhabdoid tumor market.

-pipeline.png&w=256&q=75)

