Neurofibromatosis Type 2 Market
- According to DelveInsight’s estimates, in 2023, there were approximately 17 thousand diagnosed prevalent cases of Neurofibromatosis Type-2 in the 7MM. Of these, the US accounted for approximately 62% of the cases, EU4 and the UK countries accounted for around 33%, followed by Japan which represented nearly 5%.
- The Neurofibromatosis Type 2 market is set for steady growth, with a robust compound annual growth rate (CAGR) anticipated from 2024 to 2034. This expansion in the 7MM is driven by the introduction of innovative therapies such as Brigatinib, REC-2282, and VT3989 as well as growing awareness and diagnosis, and advancements in genetic testing.
- A major unmet need in Neurofibromatosis Type 2 is the lack of FDA-approved therapies that effectively target the underlying genetic mutations. Current treatment options are primarily limited to managing symptoms, leaving a critical gap for disease-modifying therapies that can halt or reverse tumor progression.
- A significant market barrier in Neurofibromatosis Type 2 is the limited patient population due to its rarity, which reduces the commercial incentive for pharmaceutical companies to invest in large-scale drug development. This restricts the availability of funding and resources, hindering progress in discovering and advancing novel therapies.
DelveInsight’s “Neurofibromatosis Type 2 Market Insights, Epidemiology, and Market Forecast – 2034” report delivers an in-depth understanding of Neurofibromatosis Type 2, historical and forecasted epidemiology, as well as the Neurofibromatosis Type 2 therapeutics market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The Neurofibromatosis Type 2 therapeutics market report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM Neurofibromatosis Type 2 market size from 2020 to 2034. The report also covers Neurofibromatosis Type 2 treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s potential.
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
|
|
Neurofibromatosis Type 2 Market |
|
|
Neurofibromatosis Type 2s Market Size | |
|
Neurofibromatosis Type 2 Companies |
Recursion Pharmaceuticals, Takeda Pharma, Vivace Therapeutics, AstraZeneca, Recursion Pharmaceuticals Inc., Betta Pharmaceuticals Co. Ltd., Shandong Simcere-Medgenn Bio-pharmaceutical Co. Ltd,, Novartis Pharmaceuticals, Genentech Inc., GlaxoSmithKline, AstraZeneca, and others. |
|
Neurofibromatosis Type 2 Epidemiology Segmentation |
|
Neurofibromatosis Type 2 Treatment Market
Neurofibromatosis Type 2 overview
Neurofibromatosis Type 2 is a rare genetic disorder characterized by autosomal dominant inheritance, leading to neurological and neurodegenerative features that predispose individuals to tumor development, primarily within the CNS. The condition arises from alterations in the Neurofibromatosis Type 2 gene, resulting in the dysfunction of the merlin protein, which is essential for regulating multiple signaling pathways related to contact inhibition, cellular proliferation, and growth. Clinically, Neurofibromatosis Type 2 presents with a bimodal age of onset, manifesting more severely in children, who typically exhibit the aggressive Wishart type, and in adults, who may display the less aggressive Gardner type. Common signs and symptoms include bilateral vestibular schwannomas (VS), tinnitus, hearing loss, imbalance, spinal cord compression, and associated complications such as peripheral neuropathy, cataracts, and cutaneous tumors. The multifaceted nature of Neurofibromatosis Type 2 necessitates comprehensive management strategies to address the diverse clinical manifestations and improve patient outcomes.
Neurofibromatosis Type 2 diagnosis
The diagnosis of Neurofibromatosis Type 2 is primarily based on the presence of specific clinical features, adhering to the NIH diagnostic criteria. A definitive diagnosis is established if a patient exhibits bilateral VS or has a family history indicative of Neurofibromatosis Type 2, coupled with at least one additional feature such as unilateral VS, another schwannoma, meningioma, glioma, neurofibroma, or juvenile posterior subscapular lens opacity. Diagnostic tests often include advanced imaging techniques, such as MRI, which is crucial for visualizing CNS tumors and assessing their size and location. Genetic testing for mutations in the Neurofibromatosis Type 2 gene can further confirm the diagnosis and facilitate genetic counseling, particularly in familial cases. Routine audiological assessments and ophthalmologic examinations are recommended to monitor for auditory and ocular complications, respectively, enhancing early detection and intervention strategies.
Further details related to country-based variations are provided in the report...
Neurofibromatosis Type 2 treatment
The treatment of Neurofibromatosis Type 2 aims to alleviate disabling symptoms and enhance overall survival, yet there are currently no FDA-approved therapies specifically for this condition. Surgical resection remains the primary intervention for managing growing tumors; however, it poses significant morbidity risks. Radiotherapy is typically avoided due to concerns regarding secondary malignancies and the potential for malignant transformation. Advances in understanding the molecular biology of Neurofibromatosis Type 2 have illuminated its etiology, revealing several potential molecular targets for therapeutic intervention, particularly concerning central nervous system lesions. Emerging biologically targeted therapies show promise, focusing on the dysfunction of the merlin protein, which is crucial in regulating key intracellular signaling pathways, including VEGF, MEK1/2, and mTOR.
Neurofibromatosis Type 2 Epidemiology
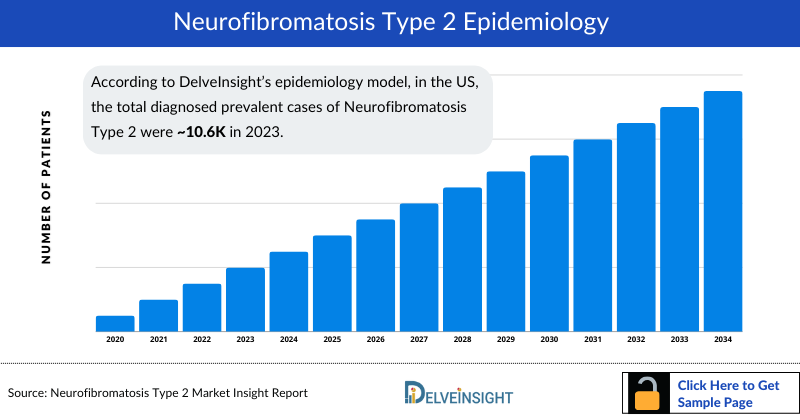
As the market is derived using a patient-based model, the Neurofibromatosis Type 2 epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by total diagnosed prevalent cases of Neurofibromatosis Type 2, age-specific diagnosed prevalent cases of Neurofibromatosis Type 2, and tumor-specific diagnosed prevalent cases of Neurofibromatosis Type 2 in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
- According to DelveInsight’s epidemiology model, in the US, the total diagnosed prevalent cases of Neurofibromatosis Type 2 were approximately 10.6 thousand in 2023. This number is anticipated to rise during the forecast period (2024-2034), driven by increased awareness and screening, along with the aging population.
- Among the EU4 and the UK, Germany accounted for the highest number of diagnosed prevalent cases of Neurofibromatosis Type 2, with approximately 1,359 cases in 2023, followed by the UK with approximately 1,185 cases, and France with nearly 1,139 cases.
- Among the age-specific diagnosed prevalent cases of Neurofibromatosis Type 2 in the US in 2023, the highest cases were observed in Adults with approximately 8,897 as compared to Children and Adolescents with nearly 1,695.
- In 2023, the number of diagnosed prevalent cases of Neurofibromatosis Type 2 in Japan was 813 among the 7MM.
- In 2023, tumor-specific categories included Vestibular Schwannoma (CN8-NST), Trigeminal Schwannomas (CN5-NST), intracranial meningioma, and Spinal nerve sheath tumor. Notably, Vestibular Schwannoma (CN8-NST) had the highest prevalence with around 708 cases reported in Japan.
Neurofibromatosis Type 2 Drug Chapters
Neurofibromatosis Type 2 Emerging Drugs
REC-2282: Recursion Pharmaceuticals
REC-2282 is a CNS-penetrant, orally bioavailable small-molecule pan-HDAC inhibitor in development for Neurofibromatosis Type 2-mutated meningiomas. Demonstrating good tolerability, including long-term dosing, it potentially offers reduced cardiac toxicity compared to existing HDAC inhibitors. Its unique attributes include oral bioavailability and CNS penetrance, distinguishing it from approved therapies. In June 2022, the initiation of the Phase II/III POPLAR-Neurofibromatosis Type 2 clinical trial was announced at the Children's Tumor Foundation NF Conference, evaluating REC-2282 for progressive Neurofibromatosis Type 2-mutated meningiomas. In October 2021, the FDA granted Fast Track designation for REC-2282, which also holds Orphan Drug designations from both the FDA and the European Commission for Neurofibromatosis Type 2-mutated meningiomas.
VT3989: Vivace Therapeutics
VT3989 has demonstrated efficacy as a monotherapy against tumors reliant on Hippo pathway dysfunction and shows promise in combination with other anti-cancer therapies across various tumor types. In April 2023, Vivace Therapeutics presented initial clinical data at the American Association for Cancer Research (AACR) Annual Meeting. Results from a Phase 1 study of this first-in-class TEAD autopalmitoylation inhibitor indicated that VT3989 was well tolerated, yielding durable antitumor responses in patients with advanced malignant mesothelioma and tumors associated with Neurofibromatosis Type 2 mutations. These findings, showcased during an oral plenary session at The University of Texas MD Anderson Cancer Center, emphasized VT3989's strong synergistic activity with osimertinib, particularly in enhancing tumor growth inhibition in EGFR mutant non-small cell lung cancer (NSCLC) xenograft models, including the HCC827 model known for its sensitivity to osimertinib.
|
Drug |
MoA |
RoA |
Company |
Phase |
|
REC-2282 |
Pan-Histone deacetylase (HDAC) inhibitor |
Oral |
Recursion Pharmaceuticals |
II/III |
|
VT3989 |
Hippo-YAP Signaling Pathway |
Oral |
Vivace Therapeutics |
I/II |
|
XX |
XX |
XX |
XXX |
X |
Note: Further emerging therapies and their detailed assessment will be provided in the final report...
Drug Class Insights
The treatment landscape for Neurofibromatosis Type 2 primarily involves targeted therapies, such as MEK inhibitors, which disrupt the mitogen-activated protein kinase (MAPK) signaling pathway often activated in Neurofibromatosis Type 2-related tumors. Additionally, histone deacetylase (HDAC) inhibitors are being explored for their potential to alter gene expression and inhibit tumor growth in Neurofibromatosis Type 2-mutated meningiomas. Tyrosine kinase inhibitors may also play a role, particularly in managing associated tumors by targeting aberrant signaling pathways. Emerging therapies, including TEAD inhibitors that specifically disrupt transcriptional activity linked to Hippo pathway dysfunction, represent a novel approach to address the underlying molecular mechanisms driving Neurofibromatosis Type 2. Collectively, these drug classes highlight a shift towards precision medicine in Neurofibromatosis Type 2 treatment, focusing on the unique genetic and molecular profiles of the tumors.
Continued in report...
Neurofibromatosis Type 2 Market Outlook
The management of Neurofibromatosis Type 2 presents significant challenges, primarily due to the absence of FDA-approved pharmacologic therapies and the reliance on surgical interventions for symptomatic tumors. Current treatment modalities, including surgical resection and localized radiation therapy, offer only temporary relief and are accompanied by considerable morbidity, such as hearing loss and neurological deficits. As Neurofibromatosis Type 2 is characterized by the progressive development of tumors like vestibular schwannomas, meningiomas, and ependymomas, there is an urgent need for effective anti-neoplastic therapies that target the underlying molecular mechanisms of the disease. Emerging drugs such as REC-2282, an HDAC inhibitor, and VT3989, a TEAD autopalmitoylation inhibitor, hold promise in addressing Neurofibromatosis Type 2-related tumors. REC-2282 has demonstrated well-tolerated profiles with durable antitumor responses in early clinical trials, while VT3989 has shown efficacy as a monotherapy and in combination with other treatments, particularly against tumors associated with Hippo pathway dysfunction. These developments highlight a shift towards precision medicine in Neurofibromatosis Type 2, indicating a burgeoning market for molecularly targeted therapies that could transform the treatment landscape for this challenging condition.
Continued in report...
Neurofibromatosis Type 2 Drugs Uptake
This section focuses on the uptake rate of potential drugs expected to be launched in the market during 2020–2034. For example, REC-2282 is expected to enter the US market in 20XX and is projected to have a XX uptake during the forecast period.
Further detailed analysis of emerging therapies drug uptake in the report...
Neurofibromatosis Type 2 Pipeline Development Activities
The report provides insights into Neurofibromatosis Type 2 clinical trilas within Phase III, Phase II, and Phase I. It also analyzes key players involved in developing targeted therapeutics.
Pipeline development activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for emerging therapies for Neurofibromatosis Type 2.
KOL Views
To keep up with current market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on Neurofibromatosis Type 2 evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake, along with challenges related to accessibility, including Medical/scientific writers, Medical Professionals, Professors, Directors, and Others.
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Centers like the Massachusetts General Hospital, USA; Department of Radiation Oncology, United States; Children's Tumor Foundation among others, were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or Neurofibromatosis Type 2 therapeutics market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Physician’s View
As per the KOLs from the US, Currently, there is no cure for Neurofibromatosis Type 2, and treatment primarily focuses on managing various symptoms. In patients with optic nerve involvement, regular examinations by the ophthalmology department are recommended. If there is a rapid decline in vision, referral to the hematology-oncology department may lead to chemotherapy as a treatment option. Overall, targeted drug options remain limited.
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyzes multiple emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
To analyze the effectiveness of these therapies, have calculated their attributed analysis by giving them scores based on their ability to improve atrial and ventricular dimension/function and ability to regulate heart rate.
Further, the therapies’ safety is evaluated wherein the adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials, which directly affects the safety of the molecule in the upcoming trials. It sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Market Access and Reimbursement
The high cost of therapies for the treatment is a major factor restraining the growth of the global drug market. Because of the high cost, the economic burden is increasing, leading the patient to escape from proper treatment.
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Further details will be provided in the report...
Scope of the Neurofibromatosis Type 2 Market Report
- The report covers a segment of key events, an executive summary, and a descriptive overview of Neurofibromatosis Type 2 explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines have been provided.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current treatment landscape.
- A detailed review of the Neurofibromatosis Type 2 therapeutics market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies by understanding trends through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM Neurofibromatosis Type 2 market.
Neurofibromatosis Type 2 report insights
- Neurofibromatosis Type 2 Patient Population
- Neurofibromatosis Type 2 Therapeutic Approaches
- Neurofibromatosis Type 2 Pipeline Analysis
- Neurofibromatosis Type 2 Market Size and Trends
- Existing and Future Market Opportunity
Neurofibromatosis Type 2 report key strengths
- 11 years Forecast
- The 7MM Coverage
- Neurofibromatosis Type 2 Epidemiology Segmentation
- Key Cross Competition
- Conjoint analysis
- Neurofibromatosis Type 2 Drugs Uptake
- Key Neurofibromatosis Type 2 Market Forecast Assumptions
Neurofibromatosis Type 2 report assessment
- Current Neurofibromatosis Type 2 Treatment Practices
- Neurofibromatosis Type 2 Unmet Needs
- Neurofibromatosis Type 2 Pipeline Product Profiles
- Neurofibromatosis Type 2 Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
- Neurofibromatosis Type 2 Market Drivers
- Neurofibromatosis Type 2 Market Barriers
Key Questions Neurofibromatosis Type 2 Market Report:
Neurofibromatosis Type 2 Market Insights
- What was the total market size of Neurofibromatosis Type 2, the market size of Neurofibromatosis Type 2 by therapies, and market share (%) distribution in 2020, and what would it look like by 2034? What are the contributing factors for this growth?
- How will REC-2282 affect the treatment paradigm of Neurofibromatosis Type 2?
- Which drug is going to be the largest contributor by 2034?
- What are the pricing variations among different geographies for approved and marketed therapies?
- How would future opportunities affect the market dynamics and subsequent analysis of the associated trends?
Neurofibromatosis Type 2 Epidemiology Insights
- What are the disease risks, burdens, and unmet needs of Neurofibromatosis Type 2? What will be the growth opportunities across the 7MM with respect to the patient population pertaining to Neurofibromatosis Type 2?
- What is the historical and forecasted Neurofibromatosis Type 2 patient pool in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan?
- Out of the countries mentioned above, which country would have the highest diagnosed prevalent Neurofibromatosis Type 2 population during the forecast period (2024–2034)?
- What factors are contributing to the growth of Neurofibromatosis Type 2 cases?
Current Neurofibromatosis Type 2 Treatment Scenario, Marketed Drugs, and Emerging Therapies
- What are the current options for the treatment of Neurofibromatosis Type 2? What are the current clinical and treatment guidelines for treating Neurofibromatosis Type 2?
- How many companies are developing therapies for the treatment of Neurofibromatosis Type 2?
- How many emerging therapies are in the mid-stage and late stage of development for treating Neurofibromatosis Type 2?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- What is the cost burden of current treatment on the patient?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the accessibility issues of approved therapy in the US?
- What is the 7MM historical and forecasted market of Neurofibromatosis Type 2?
Reasons to Buy Neurofibromatosis Type 2 Market Forecast Report
- The report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the Neurofibromatosis Type 2 market.
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- The distribution of historical and current patient share is based on real-world prescription data in the US, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
- Identifying upcoming solid players in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of Access and Reimbursement policies for Neurofibromatosis Type 2, barriers to accessibility of approved therapy, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.