How 3D Bioprinting Technology is Revolutionizing the Healthcare Industry?
May 04, 2022
Table of Contents
3D printing or additive manufacturing is the method of constructing a 3D object from a digital file. The technique is in use for several years and had a strong response from industries such as manufacturing, the art & jewelry industry, aerospace (or astrospace), construction, home development, soft sensors (and actuators), automotive, firearms, computers (and robots), among several others. Along with other sectors, in recent years, exciting development in 3D bioprinting is being observed in the healthcare industry as well. The growing research and development in 3D bioprinting are bringing huge possibilities in segments such as tissue engineering, drug delivery, cell/tissue and organ products, and other biomedical applications.
Bioprinting (also known as 3D bioprinting) is a combination of 3D printing with biomaterials to replicate parts such as natural tissues, ligaments, bones/joints, and blood vessels in the body. Apart from these, 3D bioprinting has its application in dentistry, imitation of medical devices, fabrication of anatomical models, and drug research, formulations, and development.
Downloads
Article in PDF
Recent Articles
3D bioprinting in terms of technology can be subdivided into four major types namely Magnetic Levitation, Inkjet bioprinting, Syringe-based, and Laser-based bioprinting. The most common types of material used are living Cells, extracellular matrices, hydrogels, and others. 3D bioprinting has its uses in several clinical applications and research activities.
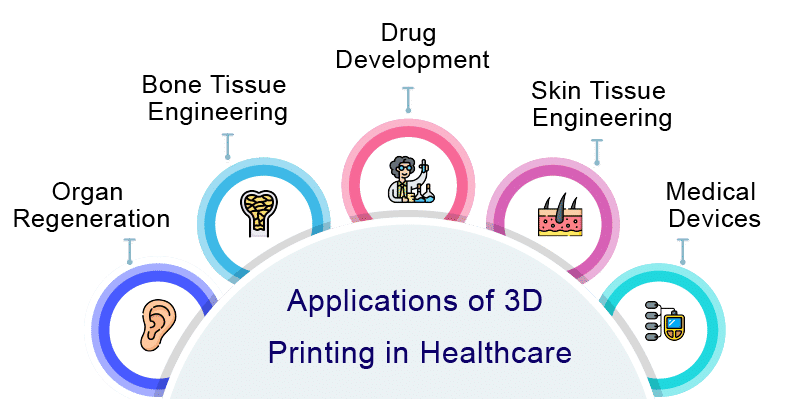
Some of the key applications of 3D bioprinting in the healthcare industry are as follows –
Bioprinting on Chip
Bioprinting is a new technology for constructing scaffolds for tissue growth. Microfluidic organs-on-a-chip is a helpful platform with several uses, mostly in drug screening and pathological research. Organ-on-a-chip models are designed to replicate the structural, microenvironmental, and physiological activities of human organs. Because of its capacity to print several materials and cell types concurrently with excellent spatial resolution and repeatability, bioprinting has recently been used to create organ-on-a-chip models. This allows for the building of a biomimetic microenvironment with a variety of 3D shapes. A functional vascularized tissue structure may be directly printed, allowing fluid flow for nutrition transfer, gas exchange, and waste elimination.
The majority of organ-on-a-chip technologies are designed for mechanistic investigations and proof-of-concept drug testing. These platforms frequently need the employment of advanced microfabrication methods. Bioprinting technology has the ability to automate these procedures and alleviate the throughput and reproducibility challenges that traditional organ-on-a-chip systems encounter. To reproduce the architecture of genuine tissue and organs, organ-on-a-chip models sometimes include 3D microchannels and intricate features. The quality and microstructure of soft scaffolds, on the other hand, are difficult to manage. This might also be addressed by bioprinting, which allows for fine-tuning of the mechanical properties, porosity, microstructure, and polymerization processes of hydrogel scaffolds.
3D printing technology fits well in the organ on a chip domain and has the potential to bridge major gaps in tissue engineering. Various tiny organ models, including the liver, heart, vasculature, and kidney, have recently been produced (i.e., printed). Furthermore, human cell-based organoids have emerged as potential tools for drug screening, personalized therapy, and disease modeling. Using these organoids as building blocks in 3D bioprinting might allow for greater scale deposition of these tissue structures. The combination of 3D bioprinting, 3D cell culture, microfluidics, and organ-on-a-chip has the potential to enable the integration of many organoids inside a single system with small footprints and better biosensing capacity.
Bioprinting and Bone Grafts
Surgery and bone grafting are considered critical pillars for healing when considerable volumes of bone are lacking. A graft that does not mimic the original form of the bone will have a detrimental impact on the success of facial reconstructive surgery. Therefore, establishing an acceptable scaffold material to replace bone, as well as an adequate procedure, has been the focus of continuing research. As a result, bone tissue engineering has piqued scientists’ curiosity since the 1980s. Its goal is to create bone graft alternatives by employing a scaffold made of a biocompatible substance that contains the osteogenic cells required for healing as well as growth factors that aid in osteodifferentiation and angiogenesis. Many biomaterials have been developed and tested since then in order to meet the requirements for safety, adequate mechanical properties, and designs that mimic the replaced bone.
However, the manufacturing advancements proceeded, and additive manufacturing (AM), often known as three-dimensional (3D) printing, was introduced into industry and biology. Rather than removing from an ingot, AM technology fabricates the prototype or scaffold by depositing bioink layer by layer. Bioink is a material that is placed with precision and polymerizes or cross-links either before or after deposition to create the scaffold. It resembles the extracellular matrix with cells enclosed within. Previously, the technology was utilized to deposit a single bioink, but as the field has advanced, multicomponent bioinks may now be deposited with great precision to imitate the intricate architecture of human tissue. Recently, CAD technology combined with improvements in AM and 3D bioprinting technology has resulted in the production of scaffolds for tissue regeneration that have been translated and implanted in patients for the repair of blood vessels, urethra, urinary bladder, and trachea.
Bioprinting and Regeneration of Skin Tissue
Burns are a prominent cause of trauma, and the focus of patient care has evolved from survival to facilitation of improved functional outcomes throughout the years. Typically, burn therapy entails surgical removal of affected skin and restoration of the burn injury with the use of skin substitutes, especially in the case of severe burn injuries. Traditional skin substitutes lack all skin cell types and do not allow for the recapitulation of native skin physiology. However, 3D bioprinting has shown promise in wound healing and regeneration in recent years. 3D bioprinting may be customized for skin form, with cells and other elements distributed accurately, resulting in speedy and dependable manufacture of bionic skin replacements that match clinical and industrial standards. It also offers exceptional performance with high resolution, flexibility, repeatability, and throughput, indicating tremendous promise for tissue-engineered skin fabrication. Three-dimensional (3D) bioprinting for burn injury regeneration entails layer-by-layer deposition of cells and scaffolding materials over the wounded regions. Skin bioprinting may be performed both in situ and in vitro. Except for the printing place and tissue maturation, both techniques are comparable. There are technological and regulatory hurdles to overcome before clinical translation of bioprinted skin for burn restoration can begin. However, the application of bioprinting for skin repair after burns is promising; bioprinting will enable exact and repeatable insertion of cell types as well as precise and reproducible manufacturing of constructs to replace the wounded or damaged locations. Overall, 3D bioprinting is a game-changing technology, and its application in wound restoration will result in a paradigm change in patient outcomes.
Challenges and Limitations of Bioprinting
The ultimate goal of 3D bioprinting is to create a technology capable of producing 3D functioning complex organs as a source for tissue grafts, full-organ transplants, and animal-alternative models for drug testing. This technology is still in its early stages, but it is fast progressing, with a multitude of research being done on bioprinting technology ranging from printing engineering to tissue engineering and cell sciences.
Despite substantial development and several breakthroughs, bioprinting technology is still facing several critical hurdles that are preventing bioprinting structures from being scaled up to viable and functioning tissues. The capacity to print an intra-organ vascular hierarchical network, from arteries and veins down to capillaries, is the most difficult problem, as tissues would not survive without it.
Biomaterials are important in 3D bioprinting because they support the structural and functional aspects of the 3d printing tissue while also preserving structural integrity and biocompatibility throughout tissue printing and maturation. However, existing printed materials are incapable of adequately imitating natural ECM compositions to sustain cellular structure. As a result, it is critical to create novel printable biomaterials that can be printed alongside live cells and have acceptable mechanical qualities for cell handling.
Another significant obstacle is cell procurement, as tissue printing necessitates a high quantity of cells. Because bioprinting influences stem cell development at several phases of the process, stem cell sources would be the most promising option. Another current restriction of 3D bioprinting is the high cost and limited throughput. All current techniques need manual cell seeding and bioink loading, whereas high-throughput 3D model manufacturing is desired. 3D organoids might be a useful large-scale screening technique for drug development in the future. However, organoids are still created on a modest scale on tissue culture plates. To solve these difficulties, trans-disciplinary research including cell biologists, engineers, physiologists, and pharmaceutical industry partners is required to enable and push the frontiers of this technology.
Companies in Bioprinting
Today, 3D Bioprinting is not limited to academic institutions and research centers, in fact, several startups also entering the segment and working to tap the commercialization possibility. As per DelveInsight’s assessment, globally more than 120+ 3D bioprinting companies are actively operating in the market. The active participation of the startup has stimulated the R&D activities in the domain and taken it to a new height. The intense R&D activities are opening new doors for regenerative medicine with the discovery of novel applications across different disciplines. As of now, only a few companies have launched commercial tissue engineering products in the market, however, in the coming years, several products are expected to launch in the market.

Organovo is one of the well-known and established leaders in the 3D bioprinting market. It is actively operating in the tissue engineering segment and developing a line of human tissues for use in medical research and drug discovery. Similarly, the other major players in the 3D Bioprinting market include Allevi, Cellink, Aspect Biosystems, 3D Systems Corporation, Cyfuse Biomedical, Envisiontec, Poietis, TeVido BioDevices, Nano3D Biosciences, Digilab, RegenHU, GeSIM GmbH, Advanced Solutions Life Sciences, Regenovoj, ROKIT Healthcare, and Regenovo Biotechnology, EnvisionTEC, Inventia Life Science PTY LTD, Vivax Bio, 3D Bioprinting Solutions, Regemat 3D, and several others. With the growing demand and rise in technology, several new players are expected to enter the 3D Bioprinting segment in the coming years.
Conclusion
It is well-established now that 3D printing can bring very promising and extraordinary results in the healthcare segment. The concept of 3D printing in healthcare is not new. The first commercial bioprinter and 3D printer development began in the late 1980s. However, the capabilities, application, and demand have picked up pace in the last few years only due to the rise in funding and advancement in technology.
With the ongoing research and development in the domain, 3D printing is getting more and more refined with each passing day. It is getting better in terms of speed, precision, features, and applications. Similarly, several key Medtech & HealthTech companies and startups are also actively working in the domain to strengthen their position. Companies are coming up with innovative applications to diversifying their portfolio and gain a competitive advantage. In order to capture the market share and enhance the manufacturing capacities over the past years, several companies have signed various partnerships, mergers, acquisitions, and collaboration deals.
As per DelveInsight, 3D printing in healthcare will grow immensely in the coming years owing to the improvement in technology, rise in chronic conditions, growing compliance in the drug development process, growth in the elderly population globally, rising demand for organs, and the active participation of the companies in the domain. Additionally, growing awareness among the general public, a rise in investment/private funding, and favorable policies by the government will also stimulate the 3D printing market growth in the coming years.
Downloads
Article in PDF