4 Late-Stage IgA Nephropathy Treatment Drugs to Look Out
Jul 22, 2024
Conventional IgA nephropathy treatments include ACE inhibitors (angiotensin-converting enzyme inhibitors) and ARBs (angiotensin II receptor blockers) to manage symptoms like high blood pressure. The only two FDA-approved treatments for managing IgAN are TARPEYO/KINPEYGO (budesonide) and FILSPARI (sparsentan). Before TARPEYO’s launch, its price level was a potential concern. However, TARPEYO has been successfully introduced at a premium price and has increased its price twice, once in early 2023 and again in early 2024.
Travere’s FILSPARI received conditional approval in February 2023. At this early stage, interest levels are moderate, as indicated by initial sales figures. This may be due to the black box warning and interest in TARPEYO. Additionally, the two-year eGFR results for FILSPARI were not significant, making it difficult for Travere to secure an unconditional label after missing the critical endpoint.
Key players in the development of IgA nephropathy therapies include Novartis (with iptacopan and atrasentan), Visterra (sibeprenlimab), Vera Therapeutics (Atacicept), RemeGen (telitacicept), among others. Omeros’s Narsoplimab failed at the interim stage, whereas Novartis is on track to have two candidates conditionally approved in 2025.
Downloads
Article in PDF
Recent Articles
- IgA Nephropathy – Navigating the Emerging Therapies and Key Companies in the Therapeutics D...
- FDA Approves Ascendis Pharma’s YORVIPATH; ARS Pharma Gets FDA Green Light for First Nasal Spray; ...
- BeiGene’s BRUKINSA Gets FDA Accelerated Approval; GSK’s Positive Results in DREAMM-8 ...
- The Expanding Market of Complement Inhibitors
- Sanofi to Acquire Provention Bio; USFDA Committee Votes in Favor Roche’s Polivy; FDA to Rev...
A leading contender in the race for IgAN treatment is Novartis’s Atrasentan, which trails TARPEYO by 2-3 years, with its 2-year filtration data expected in 2025. To compete with TARPEYO and FILSPARI, Atrasentan must demonstrate superior eGFR results. Additionally, Vera’s Atacicept is emerging as a highly promising challenger, bolstered by its positive Phase IIb results.
Here, take a closer look at four late-stage therapies that will transform the IgAN treatment space.
Novartis’ Atrasentan
Atrasentan is a potent and selective inhibitor of the endothelin A receptor (ETA). It offers potential benefits for various chronic kidney diseases by reducing proteinuria and exhibiting direct anti-inflammatory and anti-fibrotic effects to maintain kidney function. It is currently under evaluation in a Phase III registration trial (ALIGN) for IgA nephropathy and a Phase II basket trial (AFFINITY) for primary glomerular diseases, including IgAN, FSGS, and Alport Syndrome.
Recently, at the 61st European Renal Association Congress during a late-breaking clinical trials session, Novartis presented results from a pre-specified interim analysis of the Phase III ALIGN study of atrasentan. Patients receiving atrasentan, alongside supportive care (maintaining the highest tolerated and stable dose of a renin-angiotensin system [RAS] inhibitor), experienced a 36.1% reduction in proteinuria (measured by the 24-hour urine protein to creatinine ratio [UPCR]) at 36 weeks compared to those on placebo plus supportive care (p<0.0001). The study also indicated that atrasentan has a favorable safety profile, consistent with previously reported data.
Reduction in proteinuria is a recognized surrogate marker for delaying the progression to kidney failure and has been used as an endpoint in IgAN clinical trials to support accelerated regulatory approvals. The FDA submission for atrasentan in IgAN is scheduled for the first half of 2024.
The ALIGN study is ongoing in a blinded fashion, so only limited interim analysis results are available. The final analysis, which includes the key secondary endpoint of change from baseline in estimated glomerular filtration rate (eGFR) at 136 weeks, as well as the outcomes for participants receiving a sodium-glucose co-transporter-2 (SGLT2) inhibitor as background care in an exploratory cohort, is anticipated in 2026.
Atrasentan could significantly change the management of IgAN for many patients living with this complex condition, stated David Soergel, M.D., Global Head of the Cardiovascular, Renal, and Metabolism Development Unit at Novartis.
Otsuka/Visterra’s Sibeprenlimab
Sibeprenlimab (formerly known as VIS649) is an experimental humanized IgG2 monoclonal antibody designed to decrease the production of Gd-IgA1 by targeting a signaling molecule called APRIL (A Proliferation-Inducing Ligand). APRIL is thought to drive the production of IgA and Gd-IgA1. By binding to and neutralizing APRIL, Sibeprenlimab may lower IgA and Gd-IgA1 levels, which could lead to reduced auto-antibody production, fewer immune complexes, less immune complex deposition in the kidneys, and decreased kidney inflammation. This reduction in Gd-IgA1 production is believed to help prevent further kidney damage and the progression to end-stage kidney disease.
In February 2024, Otsuka and Visterra Inc. announced that the FDA has granted Breakthrough Therapy designation to their investigational drug, sibeprenlimab, for the treatment of immunoglobulin nephropathy. This designation was awarded based on positive outcomes from the Phase II ENVISION clinical trial, which assessed sibeprenlimab in patients with IgAN. Additionally, the trial results have been published in The New England Journal of Medicine.
Sibeprenlimab is still an investigational drug and has not been approved by the FDA or any other regulatory bodies. Its efficacy and safety are currently being evaluated in clinical studies.
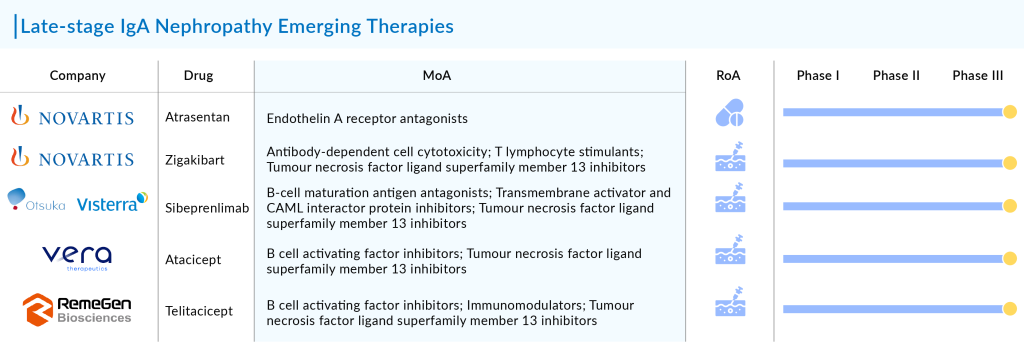
Vera Therapeutics’ Atacicept
Atacicept, developed by Vera Therapeutics, is an experimental recombinant fusion protein that includes the soluble TACI receptor, which binds to BLyS and APRIL cytokines. Following positive outcomes from the Phase IIb ORIGIN trial, the company initiated the pivotal Phase III (ORIGIN 3) trial for atacicept in June 2023 to treat patients with IgA nephropathy.
At the 61st European Renal Association Congress (ERA24) being held in Stockholm, the company presented data from Phase IIb ORIGIN trial of atacicept in immunoglobulin A nephropathy, showing that atacicept stabilized kidney function through 72 weeks and led to rapid improvements in hematuria.
Participants who received atacicept for 72 weeks maintained stable eGFR and experienced consistent, sustained reductions in Gd-IgA1, hematuria, and UPCR. Those who switched from placebo to atacicept also showed stable eGFR and similar reductions in Gd-IgA1, hematuria, and UPCR compared to participants initially randomized to atacicept during the first 36 weeks of the trial. The cumulative safety profile of atacicept remained comparable to the randomized period, with a 91% retention rate over 72 weeks. The Company believes these findings support atacicept’s potential for long-term, comprehensive IgAN disease modification and endorses the ongoing pivotal Phase III ORIGIN 3 trial of atacicept in IgAN.
RemeGen’s Telitacicept
Telitacicept, developed independently by RemeGen, is the world’s first and first-in-class recombinant B lymphocyte stimulator (BLyS) and a proliferation-inducing ligand (APRIL) dual-target novel fusion protein product for injection. It has eight indications in the field of autoimmune diseases, either commercialized or in late-stage clinical trials. Notably, the systemic lupus erythematosus (SLE) indication was approved for domestic sale in March 2021 and included in the National Reimbursement Drug List (NRDL) later that year. The drug, featuring a new target, structure, and mechanism, has been granted invention patents in China, the US, Europe, and other regions.
In summary, it is anticipated that the IgAN treatment field will undergo substantial changes from 2024 to 2034 due to the introduction of new therapies. Previously, there were no specific treatments for IgAN, and there is a critical need for therapies that prevent progression to ESKD, the main unmet need currently. Any major advancements in this area are expected to dramatically influence the IgAN treatment market during the forecast period.

Downloads
Article in PDF
Recent Articles
- FDA Approves Ascendis Pharma’s YORVIPATH; ARS Pharma Gets FDA Green Light for First Nasal Spray; ...
- Travere’s FILSPARI Approval Sparks Rivalry in the IgA Nephropathy Space
- Roche’s Columvi Phase III STARGLO Trial; Novartis’ Fabhalta Latest Data; Vertex’s Alpine Im...
- Sanofi to Acquire Provention Bio; USFDA Committee Votes in Favor Roche’s Polivy; FDA to Rev...
- Biogen’s SPINRAZA Phase II/III Trial Results; Travere’s FILSPARI FDA Approval; GSK’s ...