5 Emerging Cell and Gene Therapies That Could Revolutionize Parkinson’s Disease Treatment
Nov 25, 2024
Table of Contents
Cell and gene therapies are transforming Parkinson’s treatment, bringing new hope to patients who previously had few options. While traditional approaches focus on symptom management, these advanced therapies target the underlying causes of the disease. By introducing healthy cells or repairing faulty genes, researchers are pioneering solutions that could slow, stop, or potentially reverse Parkinson’s progression. This era of precision medicine is expanding the limits of what was once believed achievable, envisioning a future where Parkinson’s no longer controls patients’ lives.
5 Emerging Cell and Gene Therapies on the Horizon
Cell and gene therapies are revolutionizing treatments for Parkinson’s disease, offering new possibilities where traditional methods have struggled. By addressing Parkinson’s at its genetic and cellular roots, these therapies hold the potential to slow, halt, or even reverse the progression of this challenging neurodegenerative condition. Cutting-edge technologies like CRISPR, along with regenerative cell therapies, are creating opportunities to repair or replace damaged neurons, which could restore motor function and significantly enhance patients’ quality of life. The future of Parkinson’s disease treatment is shifting from merely managing symptoms to directly targeting the disease itself.
Downloads
Article in PDF
Recent Articles
- Regeneron’s I-O Drug; Gene Therapy for Parkinson’s Disease; Yumanity and Merck Inks $...
- Acceleron Pharma’s drug sotatercept touches the phase 2 mark; GT Medical receives nod for G...
- AbbVie, Sofinnova back; Gilead, Kite ink; Biogen aims filing; Reversing diabetes
- Most Promising Therapies in the Parkinson’s Disease Treatment Market
- Risvodetinib: A Promising Breakthrough in Parkinson’s Disease Treatment
The promise of cell and gene therapies for Parkinson’s is already evident in early-stage clinical trials, where initial results are inspiring optimism. As these treatments advance from research to clinical practice, they are transforming the approach to Parkinson’s care from symptom relief to actual disease modification. Patients in these trials are experiencing improved motor function and quality of life, fueling enthusiasm among healthcare providers and scientists alike.
The outlook for cell and gene therapies for Parkinson’s disease is bright. The current cell and gene therapies in Parkinson’s disease pipeline is robust, featuring a range of emerging treatments such as MeiraGTx’s AAV-GAD, Hope Biosciences’ HB-adMSCs, Sumitomo Pharma’s CT1-DAP001/DSP-1083, Prevail Therapeutics/Eli Lilly’s PR001 (LY3884961), and BlueRock Therapeutics’ Bemdaneprocel (BRT-DA01), among others. Now, let’s take a look at the 5 most promising cell and gene therapies poised to reshape the Parkinson’s disease treatment landscape.
MeiraGTx’s AAV-GAD
AAV-GAD is an experimental gene therapy aimed at reprogramming malfunctioning brain circuits by locally producing GABA, a neurotransmitter that can help restore normal activity in essential brain cells affected by any form of Parkinson’s disease. This therapy is delivered in a single infusion through a minimally invasive procedure, utilizing a proprietary device from MeiraGTx. This device administers a tiny dose—about one drop—of the gene therapy solution directly into the subthalamic nucleus, a crucial regulator of the movement-related brain circuits.
Recently in October 2024, MeiraGTx reported top-line results from its clinical bridging study, MGT-GAD-025, which investigates AAV-GAD as a treatment for Parkinson’s disease. The MGT-GAD-025 study is a six-month, randomized, three-arm, double-blind, sham-controlled trial utilizing the AAV-GAD drug, produced by MeiraGTx at its in-house facilities with their commercial-grade manufacturing process. Participants included individuals with idiopathic Parkinson’s disease who had shown a positive response to levodopa for at least a year and had a UPDRS Part 3 score of 25 or higher in the “off” state. Fourteen subjects were divided into three groups: high dose (n=5), low dose (n=5), and sham (n=4).
Participants received either AAV-GAD infused bilaterally into the subthalamic nucleus or a sham procedure, with doses of 7.0×10^10 vg (low dose) or 21×10^10 vg (high dose) per treated individual. The study’s primary focus was on the safety and tolerability of AAV-GAD, while exploratory efficacy endpoints included the mean change in MDS-UPDRS Part 3 motor scores in the “off” state and quality of life as measured by the PDQ-39 from baseline to Week 26. Subjects completing this study are eligible to enroll in a long-term follow-up study (NCT05894343) for extended monitoring over five years post-treatment.
MeiraGTx acquired this product from Vector Neurosciences. Currently, the therapy is in Phase II clinical development for Parkinson’s disease. AAV-GAD stands out as the first gene therapy for Parkinson’s disease featuring an objective imaging biomarker that aligns with clinical improvement.
Hope Biosciences’ HB-adMSCs
Hope Biosciences’ HB-AdMSCs are mesenchymal stem cells derived from adipose (fat) tissue and refined using a proprietary cell culture platform. These pure mesenchymal stem cells, sourced from adult fat, are being developed as cell-based therapies for various conditions. Currently, Hope Bio is preparing cells for a Phase II Parkinson’s disease clinical trial, authorized by the FDA and conducted by the Hope Biosciences Stem Cell Research Foundation (HBSCRF), to evaluate the safety and effectiveness of repeated intravenous infusions of autologous adipose-derived mesenchymal stem cells in enhancing daily functioning and quality of life in individuals with Parkinson’s Disease. This randomized Phase II study aims to compare the safety and efficacy of multiple infusions of allogeneic HB-adMSCs against a placebo for Parkinson’s treatment.
Sumitomo Pharma’s CT1-DAP001/DSP-1083
DSP-1083 is currently in the Phase I/II stage of development for the treatment of Parkinson’s disease. It consists of dopamine neural progenitor cells derived from induced pluripotent stem cells (iPS cells) and is administered orally. The cell therapy is transplanted into Parkinson’s disease patients to alleviate neurological symptoms by providing dopamine, which is secreted and supplemented by the transplanted cells. These progenitor cells are derived from human induced pluripotent stem cells.
In March 2024, Sumitomo Pharma announced that the FDA had cleared its Investigational New Drug (IND) Application for a clinical trial using iPS cell-derived dopaminergic progenitor cells to treat Parkinson’s disease. The IND, submitted in February 2024, received FDA approval following a 30-day review, and preparations for the study are now underway. Cryopreserved cells will be used in the trial.
Additionally, an investigator-initiated study using non-cryopreserved cells began at the University of California San Diego School of Medicine in November 2023, while Kyoto University Hospital has been conducting a similar study in Japan since 2018.
This marks Sumitomo Pharma’s first clinical trial in the US to use allogeneic iPS cell-derived differentiated cells in the regenerative medicine and cell therapy field, which is a key focus for the company. It represents a significant step in advancing its business in this area in the US.
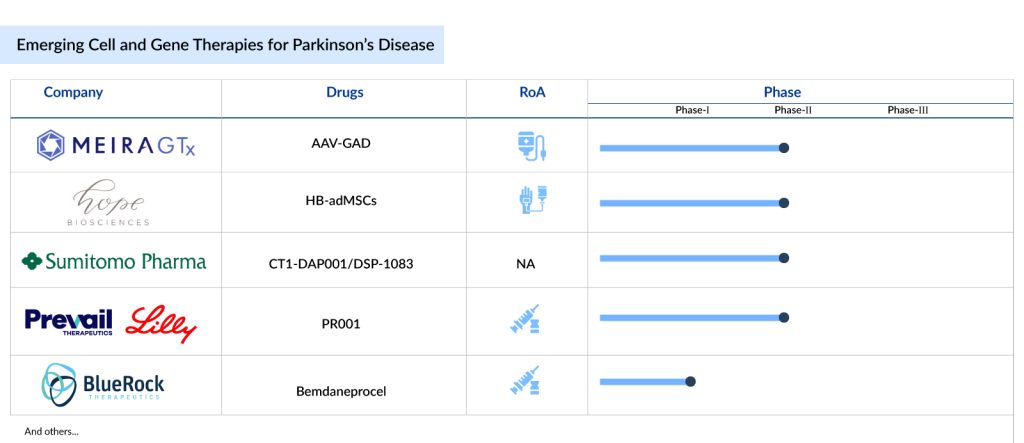
Prevail Therapeutics/Eli Lilly’s PR001 (LY3884961)
PR001 is being developed as a potential disease-modifying, single-dose gene therapy for individuals with Parkinson’s disease associated with GBA1 mutations (PD-GBA). PD-GBA is caused by mutations in the GBA1 gene, which provides the instructions for producing the lysosomal enzyme beta-glucocerebrosidase (GCase). GCase is essential for breaking down and recycling glycolipids, a type of cellular component that tends to accumulate with age. PD-GBA patients have a mutation in one copy of the GBA1 gene.
When GCase levels are insufficient, glycolipids build up, leading to lysosomal dysfunction and the aggregation of α-Synuclein protein in cells. This accumulation is believed to trigger the inflammation and neurodegeneration associated with PD-GBA and the symptoms of Gaucher disease. PR001 uses the well-established AAV9 viral vector to deliver a healthy copy of the GBA1 gene and is currently being evaluated in Phase I/II PROPEL clinical trial.
BlueRock Therapeutics’ Bemdaneprocel (BRT-DA01)
Bemdaneprocel (BRT-DA01) is an experimental cell therapy aimed at replacing the dopamine-producing neurons lost in Parkinson’s disease. The dopaminergic neuron precursors used in this therapy are derived from human embryonic stem cells. During a surgical procedure, these neuron precursors are transplanted into the brain of a Parkinson’s patient. Once implanted, they have the potential to rebuild damaged neural networks and help restore both motor and non-motor functions. However, Bemdaneprocel has not yet been approved by any health authority for the treatment of any disease or medical condition.
In May 2024, BlueRock Therapeutics announced that its investigational therapy, bemdaneprocel, for treating Parkinson’s disease received Regenerative Medicine Advanced Therapy (RMAT) designation from the FDA. Earlier, in March 2024, the company shared 18-month data from a Phase I clinical trial of bemdaneprocel. These findings were presented at the Alzheimer’s and Parkinson’s Diseases Conference in Lisbon, Portugal.
The results show that after 18 months, bemdaneprocel remains well tolerated, with no significant safety concerns. Transplanted cells survive and engraft in the brain, and the F-DOPA signal continues to rise even after immune suppression therapy was halted at 12 months, as per the study’s protocol.
Furthermore, exploratory clinical endpoints showed improvements compared to baseline in both patient cohorts, with those receiving a higher dose showing greater progress than those receiving a lower dose. These improvements were measured using the MDS-Unified Parkinson’s Disease Rating Scale Part III (MDS-UPDRS Part III) and the Hauser Diary, both of which assess the severity of motor symptoms in Parkinson’s disease.
Future Outlook of Cell and Gene Therapies in Parkinson’s Disease Treatment
The future of cell and gene therapies in Parkinson’s disease treatment is poised for significant advancements, with the potential to transform the landscape of care for patients. As research in regenerative medicine continues to progress, cell-based therapies, such as stem cell transplantation, hold promise for replacing damaged neurons and restoring motor function. Recent studies have explored the use of induced pluripotent stem cells (iPSCs) and fetal-derived dopaminergic neurons, which could help replace the lost dopamine-producing cells in the brain. These therapies aim to address the root causes of Parkinson’s disease, offering hope for long-term symptom relief and even the possibility of disease modification, rather than merely managing symptoms. Clinical trials have shown some encouraging results, though challenges remain regarding the safety, integration, and functionality of transplanted cells.
Gene therapies also offer a promising avenue for treating Parkinson’s disease by targeting the genetic and molecular mechanisms underlying the disorder. Approaches such as delivering genes that encode for neuroprotective factors, like glial cell-derived neurotrophic factor (GDNF), or correcting mutations in specific genes (e.g., LRRK2, PARK7) may slow disease progression or provide symptomatic relief. Advancements in viral vector technology, such as adeno-associated virus (AAV) vectors, have enabled more precise and efficient gene delivery to the brain. With the increasing success of these technologies in preclinical and early-stage clinical trials, gene therapies will likely become an integral part of personalized treatment regimens for Parkinson’s disease in the coming years. However, overcoming challenges related to delivery methods, long-term effects, and regulatory hurdles will be key to realizing the full potential of these therapies.

Downloads
Article in PDF
Recent Articles
- First Gene Therapy for Severe Hemophilia A; FDA Approves CellTrans’s Type 1 Diabetes Cellular The...
- Regeneron’s I-O Drug; Gene Therapy for Parkinson’s Disease; Yumanity and Merck Inks $...
- AbbVie, Sofinnova back; Gilead, Kite ink; Biogen aims filing; Reversing diabetes
- Acceleron Pharma’s drug sotatercept touches the phase 2 mark; GT Medical receives nod for G...
- Most Promising Therapies in the Parkinson’s Disease Treatment Market



