7 Companies at the Forefront of Trispecific Antibody Development
Oct 04, 2024
Table of Contents
Trispecific antibodies are the next frontier in targeted cancer therapy, offering a powerful new approach to treating complex diseases. These innovative antibodies are designed to bind to three different antigens simultaneously, enhancing treatment precision. By engaging multiple targets at once, trispecific antibodies can recruit immune cells, attack cancer cells, and block survival signals simultaneously, leading to more effective and comprehensive tumor elimination. This multi-targeted strategy not only boosts the immune response but also reduces the chances of cancer evading treatment.
What makes trispecific antibodies even more promising is their potential to revolutionize personalized medicine. By tailoring these antibodies to recognize specific markers on different types of cancer cells, they offer a more customized and precise treatment option for patients. This groundbreaking approach holds the potential to minimize side effects while maximizing therapeutic benefits, offering new hope in the fight against cancer and other complex diseases. As trispecific antibodies continue to advance, they represent a powerful tool in the evolution of immunotherapy.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- AZ offloads; Pfizer’s deal; Takeda’s work on plant; Germany’s doc payment
- Syndax Pharmaceuticals Receives FDA Approval for REVUFORJ; FDA Approves BLENREP for Adults with R...
- Kiadis inks license deal with Sanofi; Gilead Ends Hep B Collaboration; Merck & Foghorn inks ...
- Next Generation Antibody-Drug Conjugate Therapeutics and Market Analysis
- Advances in Non-Muscle Invasive Bladder Cancer Treatment: Exploring Emerging Therapies
7 Companies in the Trispecific Antibodies Arena
Bispecific antibodies have taken the spotlight, but trispecific antibodies are still relatively uncharted territory. The race to revolutionize cancer treatment is heating up in the trispecific antibodies arena, as several companies are trying their best to lead the charge.
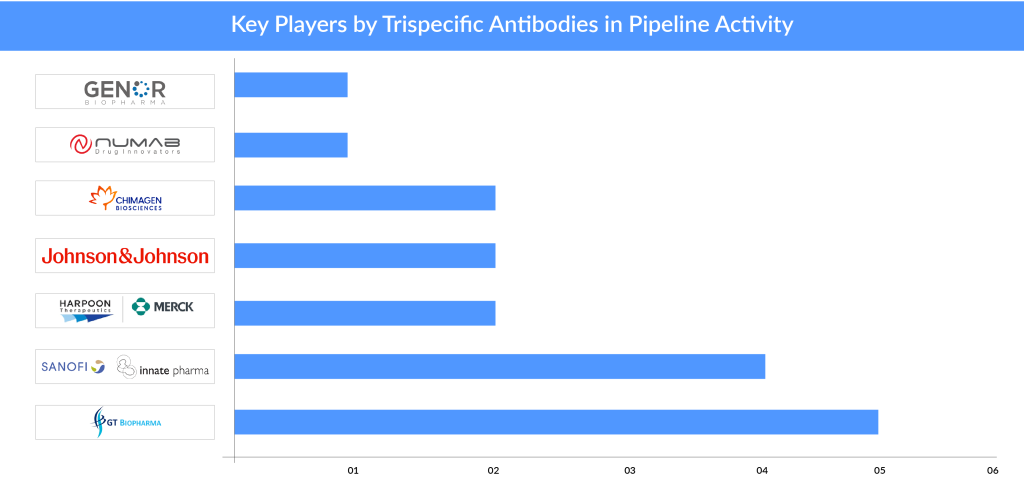
The majority of players are in the early stages of development. Notably, GT Biopharma and Sanofi in collaboration with Innate Pharma have boldly ventured into this space with more than two assets under development; in contrast, most other players have just one or two assets in their pipeline.
Let’s keep an eye on these leaders—as they’re driving the future of cancer treatment with groundbreaking approaches that may deliver game-changing outcomes.
GT Biopharma
GT Biopharma is advancing an innovative pipeline of trispecific antibodies that are designed to address various cancers by targeting multiple antigens simultaneously. One notable trispecific antibody drug in GT Biopharma’s pipeline is GTB-3550.
GTB-3550 is GT Biopharma’s first TriKE product candidate, being developed to treat AML, MDS, and other CD33+ hematologic cancers. It is a recombinant fusion protein conjugate that features single-chain, tri-specific scFv with variable regions from anti-CD16 and anti-CD33 antibodies, along with a modified form of Interleukin 15 (IL-15). The IL-15 component is a cytokine that stimulates NK cells, providing a continuous signal that activates and boosts their cancer-fighting capabilities. We plan to investigate GTB-3550 for its efficacy in treating CD33-positive leukemias, including AML, MDS, and other CD33+ hematopoietic malignancies. In April 2021, GT Biopharma revealed further interim findings from its first-in-human Phase I/II clinical trial of the GTB-3550 TriKE for treating high-risk MDS and R/R AML.
Another trispecific antibody in the pipeline is GTB-3650. GTB-3650 TriKE is the first TriKE candidate in development that incorporates camelid nanobody technology. This tri-specific molecule includes a camelid nanobody targeting the CD16 receptor on NK cells, a single-chain variable fragment (scFv) that binds to CD33 on tumor cells, and human IL-15. The FDA cleared the IND application for GTB-3650 in late June 2024.
The remaining trispecific antibodies in GT Biopharma’s pipeline include GTB-5550 (in development for the treatment of B7H3 positive solid tumor cancers), GTB-4550 (in development for the treatment of PD-L1 positive solid tumor cancers), and GTB-6550 (in development for the treatment of HER2-positive solid tumor cancers).
GT Biopharma’s trispecific antibodies represent a promising advancement in targeted cancer therapies, with the potential to significantly impact treatment paradigms and improve patient outcomes in oncology.
Sanofi/Innate Pharma
Innate Pharma has entered into a research collaboration and license agreement with Sanofi to utilize Innate’s exclusive technology ANKET (Antibody-based NK cell Engager Therapeutics) for developing innovative multi-specific antibody formats that engage NK cells via the NKp46 and CD16 activating receptors to target and destroy tumor cells.
According to the 2016 agreement, Sanofi handles the development, manufacturing, and commercialization of the resulting products, including SAR443579/IPH6101 (a trifunctional anti-CD123 NKp46xCD16 NK cell engager) and SAR445514/IPH6401 (a trifunctional anti-BCMA NKp46xCD16 NK cell engager). Under this agreement, Innate Pharma stands to receive up to €400 million in development and commercial milestone payments, in addition to royalties on net sales.
IPH6101/SAR’579 (SAR443579) is the first trifunctional anti-CD123 NKp46-CD16 NK-cell engager utilizing Innate’s exclusive ANKET multispecific antibody technology. It has demonstrated anti-tumor efficacy in pre-clinical studies, with supportive pharmacokinetic/pharmacodynamic (PK/PD) and safety data from non-human primate research, resulting in its advancement as a development candidate.
In June 2024, Innate Pharma revealed that new efficacy and safety findings from the dose-escalation phase of the Phase I/II trial involving SAR443579/IPH6101 (SAR’579) were presented in an oral session at the European Hematology Association 2024 Congress.
IPH6401/SAR’514 is a trifunctional anti-BCMA NKp46-CD16 NK-cell engager developed using Sanofi’s exclusive CROSSODILE® multi-functional platform, which features the Cross-Over-Dual-Variable-Domain (CODV) format. This engager promotes dual targeting of NK activating receptors, NKp46 and CD16, to enhance NK-cell activation, leveraging Innate’s proprietary ANKET platform.
In June 2023, SAR’579 was granted Fast Track Designation (FTD) by the US FDA for treating acute myeloid leukemia (AML). Data from the Phase I/II study, presented at EHA 2024, demonstrated that SAR’579 continues to show promising and sustained clinical efficacy with a favorable safety profile. The study has now moved into Phase II, involving 59 patients (58 with relapsed/refractory AML and 1 with high-risk myelodysplastic neoplasms) who were treated across 11 different dose levels (0.01 – 6 mg/kg). These patients had previously undergone a median of two treatments (ranging from 1 to 10).
The highest response rate was seen at a dose of 1 mg/kg weekly, with five AML patients achieving complete remission (4 complete remissions and 1 complete remission with incomplete recovery). The median duration of treatment was 7.9 weeks, with three patients experiencing durable complete remissions lasting more than 10 months, and two patients still receiving maintenance therapy at the time of the data cutoff. SAR’579 was well tolerated at doses up to 6 mg/kg weekly.
Under the license agreement made in December 2022, Sanofi acquired rights to IPH62 and IPH67, with the possibility to add one more target. According to this agreement, Innate Pharma stands to receive up to €1.35 billion in total, contingent on preclinical, clinical, regulatory, and commercial milestones, as well as royalties on potential net sales. Both these trispecific antibodies are currently in preclinical research.
Sanofi and Innate’s commitment to advancing trispecific antibody technology underscore their dedication to developing novel therapies that address unmet medical needs and offer new hope for patients with challenging conditions.
Harpoon Therapeutics (wholly-owned subsidiary of Merck)
Harpoon Therapeutics, a biotech company known for its focus on immuno-oncology, has been developing trispecific antibody therapies that leverage T-cell engagement to target and destroy cancer cells. In March 2024, Harpoon Therapeutics was acquired by Merck. As a result of this acquisition, the promising pipeline of trispecific T-cell engagers, notably through its TriTAC platform has been a part of Merck’s pipeline now.
Harpoon’s leading drug candidate, MK-6070 (formerly HPN328), is a T-cell engager that targets delta-like ligand 3 (DLL3), an inhibitory Notch ligand highly expressed in small cell lung cancer and neuroendocrine tumors. A Phase I/II clinical trial (NCT04471727) is currently assessing the safety, tolerability, and pharmacokinetics of MK-6070 as a monotherapy in select patients with advanced DLL3-expressing cancers. The trial also examines MK-6070 in combination with atezolizumab for certain SCLC patients. In March 2022, the US FDA granted MK-6070 Orphan Drug Designation for SCLC treatment.
Harpoon’s pipeline also includes HPN217, a T-cell engager targeting B-cell maturation antigen (BCMA), in Phase I trials for relapsed/refractory multiple myeloma, and preclinical candidates like HPN601, which targets epithelial cell adhesion molecule (EpCAM) for treating EpCAM-expressing tumors.
In August 2024, Daiichi Sankyo and Merck broadened their global partnership for the co-development and co-commercialization of three investigational DXd antibody-drug conjugates by adding Merck’s MK-6070. The two companies will collaborate on the development and commercialization of MK-6070 globally, except in Japan, where Merck retains exclusive rights. Merck will also handle all manufacturing and supply responsibilities for MK-6070.
Johnson & Johnson
Johnson & Johnson, through its pharmaceutical arm Janssen, has been actively advancing its trispecific antibody pipeline as part of its broader commitment to innovation in immuno-oncology and targeted therapies.
Notable candidates in their pipeline include JNJ-8543, a tri-specific antibody that redirects T-cells by targeting the CD3 protein on T lymphocytes, as well as the CD79b and CD20 surface antigens on both healthy and malignant mature B-lymphocytes.
JNJ-8543 is currently being evaluated in phase I to treat a range of hematological malignancies, including chronic lymphocytic leukemia, Waldenström macroglobulinemia (lymphoplasmacytic lymphoma), mantle cell lymphoma, follicular lymphoma, diffuse large B-cell lymphoma, marginal zone B-cell lymphoma (encompassing nodal, extranodal, and splenic types), and mucosa-associated lymphoid tissue lymphoma. This drug candidate is administered via a subcutaneous injection.
Another candidate in the pipeline is JNJ-5322. JNJ-5322 is a trispecific antibody designed to target both B-cell maturation antigen (BCMA) and G-Protein-coupled receptor class 5 member D (GPRC5D), which are strongly expressed on plasmablasts and plasma cells in multiple myeloma patients. This trispecific antibody, which includes a CD3 arm to engage T-cells, has the potential to improve tumor binding through increased avidity. This enhanced binding could lead to the effective elimination of malignant cell populations and help prevent resistance caused by the loss of tumor antigens.
These trispecific antibodies are still in early-stage clinical development but hold great potential for expanding the therapeutic landscape for cancers that have limited treatment options. Johnson & Johnson’s investment in this technology aligns with the broader industry trend of creating next-generation biologics that offer more tailored and potent treatment modalities. If successful, these therapies could provide more effective and durable responses for patients with complex and aggressive cancers, marking a significant step forward in precision oncology.
Chimagen
Chimagen is revolutionizing cancer treatment with its groundbreaking trispecific antibody pipeline. Chimagen’s lead trispecific candidate in the pipeline includes CMG1A46, being evaluated in the phase I stage of development.
CMG1A46 is a 151 kD IgG-like tri-specific antibody developed using Chimagen’s TRIAD platform. It simultaneously targets CD3 on T cells and two distinct biomarkers—CD20 and CD19—on tumor cells. This design helps recruit T cells to attack tumor cells expressing CD19 and/or CD20. CMG1A46 aims to address not only traditional CD19+CD20+ DLBCL but also CD20-CD19+ DLBCL and cases of DLBCL with low CD20 expression, thanks to its high affinity for tumor cells through its dual targeting of CD19 and CD20.
In vitro studies demonstrated that CMG1A46 facilitated the destruction of tumor cells by human PBMCs in a dose-dependent fashion, with an EC50 of approximately 0.3 pM. Compared to traditional CD3 x CD20 bispecific antibodies using a standard “1:1” IgG format, CMG1A46 exhibited greater potency and safety, as indicated by reduced target-independent T-cell activation. This antibody effectively induced significant tumor lysis in cells expressing either CD19, CD20, or both.
In vivo, 1A46 exhibited strong tumor-suppressing effects in human PBMC-engrafted NOD mouse models and triggered rapid regression of large Jeko-1 lymphoma (CD19+CD20+) and A20-hCD19 tumors (CD19+CD20-) of approximately 100mm³. Notably, CMG1A46 can be administered at a dose 6 times higher than that of standard CD3 x CD20 bispecific antibodies (3 mg/kg for CMG1A46 compared to 0.5 mg/kg for conventional ones) and demonstrated even greater anti-tumor efficacy without a significant increase in toxicity, as shown by stable animal body weight and normal cytokine levels/T cell counts in serum after the final dose. This trispecific antibody is currently in preclinical stage.
Chimagen is also working with its other assets, CMG12, a trispecific antibody with novel mechanisms designed to activate the immune system to fight colorectal cancer, a highly aggressive form of cancer. While PD-1 and CTLA4 antibodies have been beneficial for many cancer patients, there is currently no evidence showing that their effectiveness in treating colorectal cancer surpasses that of the Standard of Care, except in the dMMR subgroup.
As the pipeline advances through clinical trials, these trispecific antibody drugs could significantly reshape the landscape of targeted immunotherapy and offer new hope for patients with advanced or resistant forms of cancer.
Numab Therapeutics
Numab Therapeutics, a biotech company focused on developing innovative therapies, is advancing its pipeline with a range of trispecific antibodies designed to target multiple mechanisms simultaneously. One of the standout candidates in their pipeline is NM21-1480, a trispecific antibody designed to engage three key targets involved in cancer progression.
NM21-1480 is an advanced checkpoint modulator designed to address limitations found in current checkpoint modulator therapies. Its unique profile stems from its design as a monovalent tri-specific antibody fragment. NM21-1480 effectively blocks immune-suppressive PD-(L)1 signaling with superior potency while also stimulating 4-1BB-mediated T cell activation specifically within the tumor microenvironment, thus minimizing systemic toxicity. It was engineered to target specific epitopes with balanced affinities to enhance the combined effects of PD-L1 blockade and 4-1BB activation across patients with different levels of PD-L1 expression.
In August 2020, Numab Therapeutics AG revealed that the first patient had been treated with their proprietary ND021 program, which is an advanced tri-specific immuno-oncology strategy targeting PD-L1, 4-1BB, and Serum Albumin.
Numab’s innovative platform leverages its expertise in antibody engineering to create these sophisticated molecules, offering promising new avenues for treating cancers that are currently challenging to address with existing therapies. The development of these trispecific antibodies represents a significant advancement in the field, with the potential to significantly impact the future of targeted cancer treatment.
Genor Biopharma
Genor Biopharma is advancing its trispecific antibody platform with promising drug candidates aimed at difficult-to-treat cancers. One of their leading trispecific antibodies is GB263T, which targets EGFR and c-MET, two key proteins involved in tumor growth and drug resistance in NSCLC.
GB263T (EGFR/cMET/cMET, TsAb) is the world’s first tri-specific antibody targeting EGFR and two distinct cMET epitopes, designed to improve safety and efficacy. With its unique design, GB263T operates through multiple mechanisms, inhibiting both primary and secondary EGFR mutations as well as the cMET signaling pathway simultaneously.
In preclinical studies, GB263T outperformed its Amivantamab (JNJ-372) counterpart in preventing ligand-induced phosphorylation of EGFR and c-MET, demonstrating superior dual inhibition of the EGFR and cMET pathways. Additionally, GB263T efficiently triggered the endocytosis of EGFR and cMET, leading to a marked reduction in their protein expression. The antibody exhibited dose-dependent tumor suppression across various tumor models, including EGFR exon 20 insertions, EGFR exon 19 deletions, C797S mutations, and abnormal cMET expression. Toxicology studies in cynomolgus monkeys revealed no significant toxic side effects, even at high doses, over a four-week observation period.
Recently, in the poster session at the ESMO 2024 conference, Genor Biopharma presented updated findings from an initial phase I/II clinical trial of GB263T in patients with advanced EGFR-mutated NSCLC. In the patient group with EGFR-sensitive mutations who progressed following third-generation EGFR-TKI therapy, a confirmed objective response rate (ORR) of 28.6% was observed at effective therapeutic doses of 1260/1680 mg. Additionally, three patients with cMET alterations after third-generation EGFR-TKI treatment showed significant clinical benefit, with one patient experiencing a treatment duration of over 12 months (840 mg) at the time of data cutoff on December 31, 2023.
Future Outlook of Trispecific Antibodies
Trispecific antibodies represent the cutting edge of immunotherapy, poised to revolutionize the treatment of cancer and other complex diseases. These innovative molecules can simultaneously engage three different antigens, providing a more precise and potent approach to targeting cancer cells, stimulating immune response, and minimizing off-target effects. By combining multiple functions into a single agent, trispecific antibodies can overcome tumor resistance, improve patient outcomes, and potentially reduce the need for combination therapies, marking a new era in personalized medicine.
The future impact of trispecific antibodies is immense, offering hope for patients with difficult-to-treat cancers and other conditions. With the ability to precisely direct the immune system to multiple targets at once, these therapies may reduce treatment durations and enhance efficacy across a wide range of malignancies. Additionally, their flexible design allows for tailored approaches to different cancer subtypes, offering a new level of adaptability in therapeutic interventions. As clinical trials progress, trispecific antibodies may become a game-changer in both oncology and beyond, reshaping the future of immunotherapy.

FAQs
Key companies such as GT Biopharma, Sanofi, Innate Pharma, Harpoon Therapeutics, Merck, Johnson & Johnson (Janssen), Chimagen, Numab Therapeutics, and others are currently active in the trispecific antibody therapeutics market.
Some of the trispecific antibodies in the clinical trials include GTB-3550, GTB-3650, GTB-5550, GTB-4550, GTB-6550, SAR443579/IPH6101, SAR445514/IPH6401, JNJ-8543, JNJ-5322, CMG1A46, NM21-1480, ND021, and others.
Trispecific antibodies are positioned as the next evolution in ultra-precise immunotherapies, with the potential to transform oncology and beyond by improving efficacy, safety, and adaptability.
Downloads
Article in PDF
Recent Articles
- Merus’ BIZENGRI: The New Face of NRG1+ Cancer Treatment After FDA Approval
- AstraZeneca’s Imfinzi for Biliary Tract Cancer; FDA Clears Boehringer’s Spesolimab; Novo Nordisk ...
- Colorectal Cancer Awareness Month
- Adagio Raises $336 M; Lyndra’s Schizo Trial; BMS Opdivo for Gastric Cancer; Anixa Ovarian C...
- 5 Upcoming Bispecific & Trispecific Antibodies Beyond Oncology