7 HSV-associated Oncolytic Virus Therapies in Late and Mid-Stage
Aug 14, 2024
Table of Contents
Herpes Simplex Virus (HSV)-associated oncolytic virus therapeutics are a promising approach to cancer treatment. These treatments use genetically engineered HSV to target and destroy cancer cells while preserving healthy tissue specifically. The HSV is designed to increase selectively within tumor cells, causing their demise via viral lysis. Furthermore, these modified viruses can be programmed to express therapeutic genes that boost the immune response to the tumor. This combination action—direct oncolysis and immune system stimulation—forms a potent method for attacking cancer. Clinical trials have demonstrated that HSV-based oncolytic virus therapy can improve treatment outcomes, providing a novel and effective technique for managing various cancer types.
Approved Herpes Simplex Virus-associated Oncolytic Virus Therapies
To date, only two HSV-associated oncolytic viruses have received regulatory approval. Talimogene laherparepvec (T-VEC), marketed as IMLYGIC (Amgen), is approved in the US and Europe for the treatment of melanoma. In Japan, DELYTACT (Daiichi Sankyo) has been approved as a regenerative medicine for malignant glioma.
Downloads
Article in PDF
Recent Articles
- Is Gene Therapy the Next Cancer Treatment Revolution?
- 15 Prominent Indications for Oncolytic Virus Therapy
- 8 Late and Mid Stages Adenovirus-associated Oncolytic Virus Therapies To Watch Out
- Top 7 Emerging Vaccinia Virus-associated Oncolytic Virus Therapies
- Oncolytic Virus Cancer Therapy: Transforming Cancer Treatment
Amgen’s IMLYGIC is the first oncolytic viral immunotherapy approved for treating inoperable metastatic stage IIIB/C–IVM1a melanoma locally. Administered via direct intratumoral injection, T-VEC aims to induce both local and systemic immune responses, leading to tumor cell destruction, release of tumor-specific antigens, and activation of tumor-targeted T-cells. Its approval has sparked interest in exploring its potential combined effects with other cancer immunotherapies in both preclinical and clinical settings.
Studies have demonstrated that T-VEC can effectively work in conjunction with immune checkpoint inhibitors. T-VEC is a modified oncolytic herpes virus designed for intratumoral injection that produces granulocyte-macrophage colony-stimulating factor (GM-CSF) to boost local and systemic antitumor immune responses. The drug is also being assessed for other cancers, including breast cancer, soft tissue sarcoma, liver cancer, and solid tumors.
On the other hand, Daiichi Sankyo’s DELYTACT is a genetically modified herpes simplex virus type 1 (HSV-1) developed by Dr. Todo and his team. This oncolytic virus therapy features three mutations in its genome, leading to enhanced and targeted replication within cancer cells and a stronger anti-tumor immune response while maintaining high safety standards.
DELYTACT is the first third-generation oncolytic HSV-1 to be tested in humans. It has received conditional and temporary approval in Japan for treating malignant gliomas, based on Phase II oncolytic virus clinical trial results. Ongoing approval may depend on further evidence of clinical benefit and safety from a post-market comparative study. DELYTACT is not approved for use outside of Japan.
7 Promising HSV-associated Oncolytic Virus Therapies in Late and Mid Stages of Development
Currently, several companies including Replimune, Treovir, Immvira Pharma, Virogin Biotech, Takara Bio, Immvira Pharma, Candel Therapeutics, Sorrento Therapeutics, Shanghai Yunying Medical Technology, Mustang Bio, Hangzhou Converd Co., Ltd., Mustang Bio, BullFrog AI, and others are developing HSV oncolytic virus therapies in various stages of development.
Let’s dive deep into the most promising HSV-associated oncolytic virus therapies that are in the late and mid stages of development and have the potential to change the oncolytic virus immunotherapy landscape in the coming years.
Replimune’s RP1; RP2; and RP3
RP1: Phase III (Melanoma)
RP2: Phase II (Colorectal Cancer)
RP3: Phase II (Hepatocellular Carcinoma, Colorectal Cancer)
Replimune is currently evaluating its three lead HSV-associated oncolytic virus therapies namely RP1, RP2, and RP3 in different phases.
Vusolimogene oderparepvec, commonly known as RP1, is an HSV oncolytic virus therapy developed by Replimune Group, Inc. It acts as a CSF-2R stimulant and GALV-GP R-modulator and was originally developed to treat cancers, skin and musculoskeletal disorders, and respiratory conditions. RP1 HSV-associated oncolytic virus therapy has shown particular promise for tumors resistant to PD-1 inhibitors, as it penetrates the cells, persists in the environment, combats the disease, replicates, and continues to infect surrounding cells. Currently, RP1 is in Phase III oncolytic virus clinical trials for melanoma and is also being studied for its potential in treating skin cancer, solid tumors, and squamous cell carcinoma.
In June 2024, Replimune will begin a Phase III clinical trial to compare the effects of combining Vusolimogene Oderparepvec with Nivolumab against a treatment chosen by the physician for patients with advanced melanoma who have progressed on Anti-PD-1 and Anti-CTLA-4 therapies. The study is anticipated to conclude by January 2029 and aims to enroll approximately 400 participants.
On the other hand, RP2 is a derivative of RP1, Replimune’s leading product candidate. It uses a new, proprietary strain of herpes simplex virus that has been genetically engineered with a fusogenic protein (GALV-GP R) and GM-CSF to enhance its ability to kill tumors, stimulate the immune response against tumor cell death, and activate a systemic anti-tumor immune response.
Additionally, RP2 HSV-associated oncolytic virus therapy includes an anti-CTLA-4 antibody-like molecule, alongside GALV-GP-R and GM-CSF. Its design aims to deliver these proteins precisely to the tumor sites and surrounding lymph nodes, thereby focusing systemic immune effects on the tumors and reducing off-target toxicity. RP2 HSV-associated oncolytic virus therapy has shown early safety and anti-tumor effectiveness in patients with solid tumors. Currently, it is in Phase II clinical trials for colorectal cancer and is also being tested for other solid tumors and melanoma.
RP3 is also an advanced version of RP1 that includes additional immune-stimulating proteins. It is a selective, replication-competent oncolytic immunotherapy (TDOI) based on HSV, designed to deliver the GALV-GP-R- fusogenic glycoprotein, an anti-CTLA-4 antibody-like molecule, CD40L, and 4-1BBL. RP3 HSV-associated oncolytic virus therapy aims to precisely and effectively deliver these proteins to the tumor and the associated lymph nodes, enhancing the immune response against the tumor while minimizing unwanted side effects.
Currently, RP3 is in Phase II oncolytic virus clinical trials for treating hepatocellular carcinoma, and it is also being assessed for use in other solid tumors and colorectal cancer.
In April 2024, Replimune, in partnership with Roche, launched a Phase II open-label, multicenter trial to examine the effectiveness of RP3 oncolytic virus immunotherapy combined with first- or second-line treatments in patients with locally advanced, unresectable, or metastatic Hepatocellular Carcinoma. The study is projected to conclude by April 2027, with an anticipated enrollment of 60 participants.
Treovir’s G207
Phase II (Brain Cancer)
G207 is an advanced form of oncolytic herpes simplex virus type 1 immunotherapy that has been in development for more than 20 years. It is specifically engineered to target and destroy tumor cells while leaving healthy cells unharmed, due to the absence of the thymidine kinase (TK) and γ34.5 genes which limit its replication in normal cells. Preclinical studies have shown that G207 significantly improves survival rates in mice with both subcutaneous and orthotopic tumor models. Additionally, in a phase I clinical trial, G207 HSV-associated oncolytic virus therapy was demonstrated to be safe and well-tolerated by patients with recurrent malignant glioma, showing some potential for antitumor activity.
In June 2024, Treovir launched a Phase II Clinical Trial to evaluate the effectiveness and safety of administering a single 5 Gy radiation dose alongside intratumoral G207, an experimental viral therapy, in children with recurrent high-grade gliomas. The trial aims to assess how well this combination works and confirm its safety. Currently, the oncolytic virus clinical trial is not enrolling participants and is projected to finish by December 2028, including 40 participants.
Immvira Pharma’s MVR-T3011
Phase II (Head and neck cancer; Malignant melanoma; Sarcoma)
MVR-T3011, ImmVira’s advanced 3-in-1 oHSV, represents a cutting-edge design based on ImmVira’s expertise in oncolytic viruses and advanced gene recombinant technology. This novel genetically engineered oHSV is optimized to balance HSV-1 replication potency with safety for normal cells, enabling a breakthrough in intravenous administration while maintaining tumor selectivity. Additionally, it includes two updated, validated payloads with exogenous immune-modulating genes—PD1 antibody and IL12—to enhance antitumor effects and stimulate immune response within the tumor environment. MVR-T3011 HSV-associated oncolytic virus therapy is currently undergoing Phase II clinical trials for treating head and neck cancer, malignant melanoma, and sarcoma.
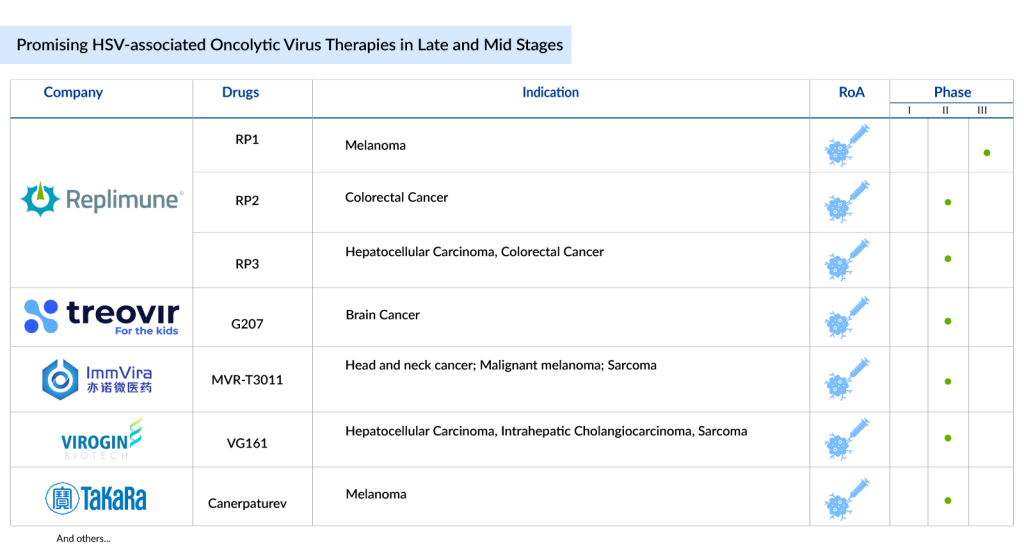
Virogin Biotech’s VG161
Phase II (Hepatocellular Carcinoma, Intrahepatic Cholangiocarcinoma, Sarcoma)
VG161 is an oncolytic virus developed using Virogin Biotech’s proprietary Synerlytic™ Platform. It is a modified HSV-1 with added payloads of IL12 and IL15/IL15Ra, as well as a unique PD-L1 blocking peptide. The virus’s neurovirulence is reduced by removing the ICP 34.5 gene. VG161 HSV-associated oncolytic virus therapy is both safe and effective in various tumor xenograft mouse models and GLP toxicity studies during preclinical phases.
In July 2023, Virogin Biotech revealed that the FDA had awarded Fast Track designation to VG161 for use in patients with advanced, unresectable Hepatocellular Carcinoma. Earlier, in February 2023, Virogin Biotech had announced that the FDA’s Office of Orphan Products Development had officially responded, granting VG161, a Class I innovative oncolytic virus product, approval for treating Intrahepatic Cholangiocarcinoma.
It is currently being explored in multiple Phase I and II oncolytic virus clinical trials in the US, Australia, and China, with a total of 10 ongoing trials (both monotherapy and combination therapy). The drug is in Phase II development for treating hepatocellular carcinoma and intrahepatic cholangiocarcinoma.
In January 2024, Virogin Biotech Canada Ltd launched an open-label, multi-center Phase IIa/IIb oncolytic virus clinical trial to assess the effectiveness, safety, and tolerability of VG161 both as a standalone treatment and in combination with Nivolumab for patients with Hepatocellular Carcinoma or Intrahepatic Cholangiocarcinoma. The trial is currently enrolling participants and is expected to finish by October 2025, with a target enrollment of 97 participants.
Takara Bio’s Canerpaturev
Canerpaturev (C-REV) is a modified version of the HSV-1 that has been engineered to fight cancer. When injected into cancerous areas, it targets and destroys tumor cells while minimizing damage to surrounding healthy tissue. This selective replication within tumors makes C-REV a promising candidate for cancer therapy. Additionally, it enhances the immune system’s response against cancer cells and helps prevent the formation of new tumors, even in untreated areas. Currently, C-REV HSV-associated oncolytic virus therapy is in Phase II clinical trials for malignant melanoma and is also being assessed for its effectiveness in treating pancreatic cancer.
What’s Ahead for HSV-associated Oncolytic Virus Therapies?
The future of HSV-associated oncolytic virus therapies looks increasingly bright due to advances in genetic engineering, which allow for precise modifications to enhance viral specificity and efficacy. Emerging strategies focus on combining these therapies with other treatments, such as immune checkpoint inhibitors or targeted therapies, to improve overall outcomes.
Apart from the late and mid-stage HSV-associated oncolytic virus therapies mentioned above, several other therapies also that are also in different stages of development. These include Candel Therapeutics’ CAN-3110; Immvira Pharma’s MVR-C5252; Sorrento Therapeutics’ STI-1386; Shanghai Yunying Medical Technology’s R130; Mustang Bio’s MB-108 and MB-109; Hangzhou Converd Co., Ltd.’s CVD 1301.H03; and BullFrog AI’s HSV-1 oncolytic virus therapeutic.
These therapies are still in the initial stages of development. However, the expected introduction of these HSV-associated oncolytic virus treatments is projected to drive growth in the oncolytic virus cancer therapy market in the near future.
Additionally, ongoing research aims to overcome challenges related to virus delivery, immune response, and tumor heterogeneity. As these approaches evolve, they hold the potential to offer more personalized, effective, and less toxic options for cancer patients, significantly advancing the field of oncology.

Downloads
Article in PDF
Recent Articles
- Oncolytic Virus Cancer Therapy: Transforming Cancer Treatment
- 15 Prominent Indications for Oncolytic Virus Therapy
- Is Gene Therapy the Next Cancer Treatment Revolution?
- Top 7 Emerging Vaccinia Virus-associated Oncolytic Virus Therapies
- 8 Late and Mid Stages Adenovirus-associated Oncolytic Virus Therapies To Watch Out