Preventing Cervical Cancer Through Screening And HPV Vaccines
Nov 08, 2024
Cervical cancer stands as the fourth most diagnosed cancer among women globally, trailing breast, colorectal, and lung cancers. However, its impact is disproportionately heavy on developing nations, where prevalence rates soar. In 2022, WHO estimated approximately 660,000 new cases of cervical cancer and 350,000 deaths worldwide, with about 80% of these cases occurring in lower- and middle-income countries. While developed nations show significantly lower rates due to preventive measures like widespread HPV vaccination and regular screenings, developing countries continue to struggle with high incidence rates and limited healthcare infrastructure.
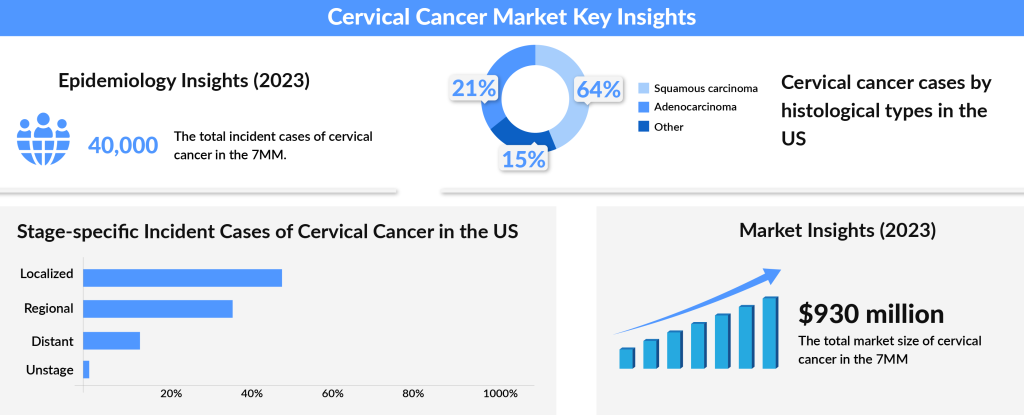
According to DelveInsight’s data, the total prevalence of cervical cancer in the 7MM (the US, EU5, and Japan) was about 40,600 in 2023, with the United States contributing nearly 34% of these cases, reflecting the highest prevalence among these regions. Within this total, squamous cell carcinoma represented roughly 9,000 cases in the US alone in 2023. Most cervical cancer diagnoses occur between the ages of 35 and 44, with the average age of diagnosis being 50. Interestingly, over 20% of cases are diagnosed in women aged 65 and older, with younger cases (under age 20) remaining rare. While numbers are expected to decline slightly over the forecast period due to continued preventive measures, the need for innovative solutions remains paramount in the global fight against cervical cancer.
The disproportionate burden is attributed to the availability of effective diagnostic technologies, running cytology-based screening tests, and quality healthcare services in developed nations, which developing countries often struggle to provide. Over the decades, governments, researchers, pharmaceutical companies, cancer advocates, policymakers, and private healthcare investors have worked tremendously to improve women’s health in developed nations.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Comprehensive Assessment of the Leading Companies in the Vaccines Market
- Widespread Usage of HPV Vaccine Reduces Cervical Cancers and Precancers
- Cervical cancer
- Cervical Cancer Treatment: Navigating the Changing Landscape
- Sanofi’s Rare Disease Drug Xenpozyme’ Approval; FDA Approves Novartis’ Pluvicto; AstraZenec...
From incoherent molecular tests to strong evidence supported by PCR, and DNA hybridization techniques, several etiological studies successfully demonstrated more than 99% of Cervical cancer cases, unlike other cancers, are not a result of genetic/ physical/ environmental factors or a combination of all, but due to the infection with types of sexually-transmitted human papillomavirus (HPV). HPV is a colossal group of over 200 related viruses. Out of these, around 40 are transmitted sexually. Besides the cancer of the cervix, Human papillomavirus can cause cancer of the vagina, vulva, penis, and anus.
With a proper understanding of the etiology of cervical cancer, screening plays a significant role in timely diagnosing and improving the overall survival rate. Worldwide, efforts have been put into implementing comprehensive screening tests to diagnose cervical cancer. However, regarding guidelines, testings, screening modalities, and implementations, almost everything differs by geography, demographics, ethnicity, quality of life, education, and healthcare status. Although the immune system flushes out HPV from the body within a span of 1-2 years, it is recommended for young, sexually active women to undergo a Papanicolaou (Pap) smear test every three years beginning at the age of 21.
For women aged 30 or older, a Pap test alone in three years, an HPV DNA screening test alone in five years, or a co-testing with both in five years is advised. While the Pap test checks for abnormal cells in the cervix, the HPV test searches for HPV in the cervix. Then, there is also a visual inspection with acetic acid done to screen for Cervical cancer. Frequent testing or follow-up screenings not only limit the scope of false-negative detection but also help detect small changes that get neglected and are potential carriers of cancers.
Thanks to the screening tests along with the HPV vaccine, today, Cervical cancer can be prevented. The HPV vaccine is a type of prophylactic cancer vaccine approved to prevent anal, cervical, vaginal, and vulvar cancer. HPV vaccines are heralded as one of the significant advances in modern oncology in the past 50 years and continue to dominate the cancer vaccines market. To date, the USFDA has approved three HPV vaccines: CERVARIX (GlaxoSmithKline; the bivalent HPV virus-like particle vaccine (2vHPV)), GARDASIL (Merck; the quadrivalent HPV virus-like particle vaccine (4vHPV)), and GARDASIL 9 (Merck; nine-valent HPV virus-like particle vaccine (9vHPV)).
GARDASIL and CERVARIX vaccines have shown remarkable effectiveness, demonstrating 100% efficacy in preventing infections with HPV types 16 and 18, which are two of the high-risk types that significantly contribute to what causes cervical cancer. Likewise, GARDASIL 9 has successfully prevented diseases linked to the four shared HPV types (6, 11, 16, and 18). Today, GARDASIL 9 has become the standard, replacing the original GARDASIL. Since the introduction of the first vaccine against HPV, data from high-income groups has shown a substantial reduction in infections with HPV and cervical cancer cases, as well as a decrease in anogenital warts and CIN2+ diagnoses among vaccinated women and girls. Although the vaccines offer protection for 6-10 years, ongoing studies are evaluating the long-term efficacy of HPV vaccination in preventing cervical cancer symptoms over a lifetime.
Pairing screening with HPV vaccination is a powerful approach in cervical cancer treatment and prevention, with screening tools now refined to help researchers understand cancer’s progression almost entirely. The launch of HPV vaccination programs for both females and males aged 9-26 years in the US, UK, and Germany has driven a significant increase in the HPV vaccine market. Signs of cervical cancer have notably decreased as vaccination rates have risen, with the market currently led by GARDASIL and CERVARIX. Of the two, GARDASIL has generally taken the lead due to its broader serotype coverage, adding to Merck’s revenue and achieving a significant milestone with its launch in China.
However, GARDASIL has faced some challenges, particularly concerning high costs and the administration of vaccines to girls at a young age, around 10 years old. Additionally, controversy arose following a trial in India that involved allegations of serious side effects and improper consent, including accusations related to eight reported deaths. This situation underscored the importance of informed awareness about HPV and cervical cancer, as well as the potential cervical cancer symptoms that HPV vaccination can help prevent. For effective cancer prevention, it is crucial that the public understands the proven benefits and safety of these vaccinations, especially given that HPV is a primary factor in what causes cervical cancer.
As demand for the HPV vaccine grows in developed countries, companies are also working to raise awareness and convince policymakers in developing nations of the vaccine’s cancer-preventing potential. The question of whether can you get cervical cancer without HPV is relevant here, as while HPV infection is the most significant cause of cervical cancer, understanding that it’s not the sole cause can help individuals make informed choices. Ultimately, symptoms for HPV cervical cancer can largely be prevented with a combination of screening and vaccination, positioning the HPV vaccine as a critical tool in reducing the global burden of cervical cancer.As we move forward in the fight against cervical cancer, the impact of preventive tools like HPV vaccination and screening cannot be overstated. These advancements are not just statistics; they represent lives saved, families kept whole, and a future where cancer prevention is within reach for millions. While there are still hurdles to overcome in making these tools accessible to everyone, the path forward is clearer than ever. With continued global efforts to spread awareness and increase access, we’re on the brink of a world where cervical cancer has become a preventable chapter in history.

Downloads
Article in PDF
Recent Articles
- Navigating the Healthcare Horizon: Odyssey of Mergers, Funding, and Acquisitions in 2024
- Insights into the Evolving Landscape of Antibody-Drug Conjugate (ADC) & the Key Companies in...
- Cervical Cancer Treatment: Navigating the Changing Landscape
- Bayer’s AskBio Initiates Phase II GenePHIT Trial; FDA Approves Merck’s KEYTRUDA Plus Chemoradioth...
- Widespread Usage of HPV Vaccine Reduces Cervical Cancers and Precancers