Revolutionizing Schizophrenia Treatment: The Key Players Shaping the Future
Oct 18, 2024
Schizophrenia is over a hundred years old term; and ever since it has kept the scientific communities scratching their head. Obvious psychotic symptoms, other neurodegenerative mimics, underdiagnosis, and sometimes even overdiagnosis, Schizophrenia is a complex disorder in itself, affecting approximately 20 million people worldwide. As per DelveInsight, the total Schizophrenia prevalent population in the 7MM (the US, EU5 (the UK, Germany, Spain, France, and Italy) and Japan) alone is expected to be 5,748,062 in 2020. The number is huge and these cases are further expected to increase during the forecast period, i.e., 2020–2030.
A chronic psychotic disorder, Schizophrenia meddles with the patient’s thoughts, interferes with a patient’s social ability, and hampers cognitive development. And a lot of research has been going on for the reception of a clearer picture of the illness. However, despite a century of research, the pathophysiology of Schizophrenia remails elusive.
Schizophrenia Market: The Present Case Scenario
At present, there is no existing cure for Schizophrenia; however, the illness is treatable and manageable with the help of a combination of therapies. Although the goals of the treatment of Schizophrenia vary with severity and phase, the short-term primary aim of Schizophrenia treatment is to manage symptoms. First and foremost, psychiatrists aim for managing psychotic symptoms and improve the quality of life (QoL) of the patients. However, the long-term goal of the treatment targets to achieve stability and prevention of relapse of the illness.
Downloads
Article in PDF
Recent Articles
- US/AZ deal; Alkermes’ Schizophrenia drug; Innovent/Lilly’s China market expansion; Re...
- 5 Upcoming Schizophrenia Drugs Competitors for Newly Approved BMS’ KarXT (COBENFY)
- Hope on the Horizon: Brilaroxazine’s Promise for Schizophrenia Patients
- Vanda Broadens its Reach to Bipolar Disorder Treatment Market With Fanapt Approval
- Breaking Boundaries: Innovations and Updates in Schizophrenia Treatment
While medications are the cornerstone of the Schizophrenia treatment market, pharmacological treatment plays an important role in managing the illness as well. The treatment options for Schizophrenia include medications (antipsychotics), psychological counseling and social support, cognitive behavioral therapy, electroconvulsive therapy (ECT). The Schizophrenia market comprises antipsychotics (APs) as the first-line treatment. These can be classified as first-generation antipsychotics (FGA; or typical APs) and second-generation antipsychotics (SGA; or atypical APs). These are either administered orally in case of oral antipsychotics (OAP) or intravenously in case of long-acting injectable therapies (LAI). Although in practice, both FGA and SGA are used in the management of Schizophrenia, however, the effectiveness of the Second-generation over the first one remains debatable, and so is of the LAI over OAP.
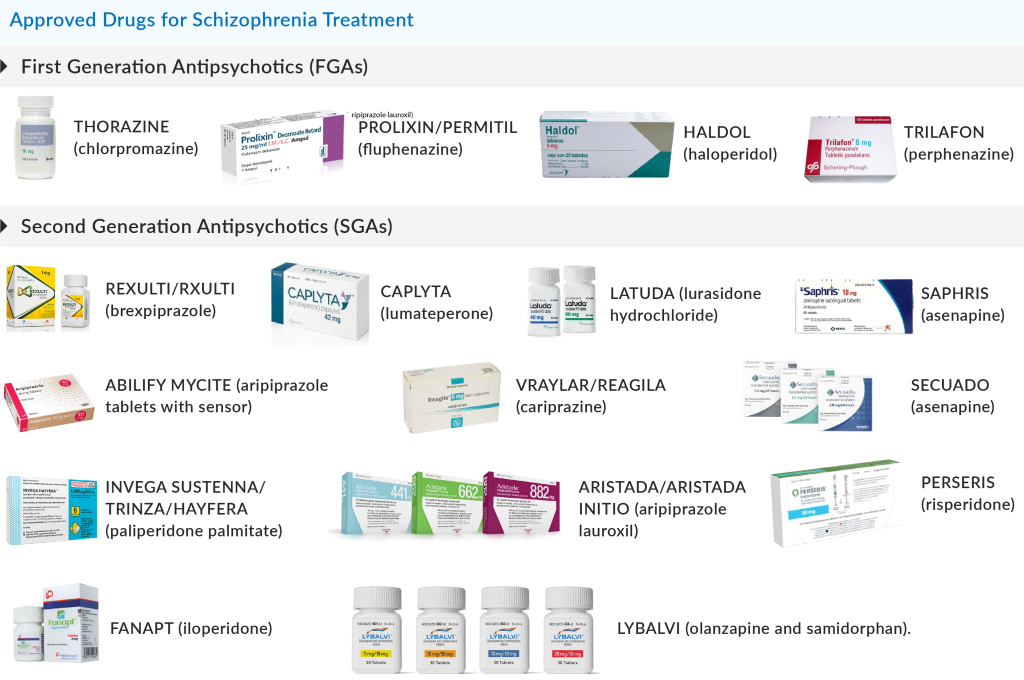
There are several FDA-approved antipsychotics available in the schizophrenia market. Some of them include REXULTI/RXULTI (brexpiprazole), CAPLYTA (lumateperone), LATUDA (lurasidone hydrochloride), SAPHRIS (asenapine), ABILIFY MYCITE (aripiprazole tablets with sensor), VRAYLAR/REAGILA (cariprazine), SECUADO (asenapine), INVEGA SUSTENNA/TRINZA/HAYFERA (paliperidone palmitate), ARISTADA/ARISTADA INITIO (aripiprazole lauroxil), PERSERIS (risperidone), FANAPT (iloperidone), LYBALVI (olanzapine and samidorphan), and several others.
Talking about the most recent approvals, on September 26, 2024, Bristol Myers Squibb announced that the FDA approved COBENFY (xanomeline and trospium chloride), an oral medication for treating schizophrenia in adults. The FDA approval is especially notable as it marks the first novel drug for schizophrenia in 35 years, introducing a new class of treatment by selectively targeting M1 and M4 receptors in the brain without blocking D2 receptors.
Wish to Know More About the Recent Approvals of Schizophrenia Drugs? Click here.
Furthermore, antipsychotic drugs are often prescribed together in combination with neuroleptics and antiepileptic for the treatment of Schizophrenia. To help patients cope with depression, which is relatively high in Schizophrenia patients, antidepressants are also prescribed. Several pieces of research have vouched for a combination of antipsychotics in combination with benzodiazepines so as to help control sleep disturbances, anxiety, or behavioral disinhibition. Anticholinergic drugs in conjunction with antipsychotics have also been identified as an important milestone in the Schizophrenia treatment market.
Schizophrenia Recent Developments
- On September 21, 2024, Teva Pharmaceuticals announced positive results from its Phase 3 SOLARIS trial for TEV-749, a subcutaneous injection for schizophrenia. The trial demonstrated significant reductions in the Positive and Negative Syndrome Scale (PANSS) total score by week 8, along with improvements in key secondary endpoints. Notably, there were no cases of post-injection delirium/sedation syndrome (PDSS). These findings were presented at the 37th Annual European College of Neuropsychopharmacology Congress in Milan, Italy, from September 21-24, 2024.
- On September 12, 2024, BMO Capital Markets analyst Evan Seigerman raised concerns about Neurocrine Biosciences’ developmental strategy following inconsistent results from mid-stage trials of luvadaxistat. Neurocrine announced it will discontinue development of the investigational schizophrenia drug after the Phase II ERUDITE study failed to meet its primary endpoint of cognitive improvement in over 200 patients. The company noted that luvadaxistat could not replicate the cognitivea benefits observed in the earlier INTERACT study, where a 50-mg dose showed significant cognitive improvements.
- In April 2024, the FDA put a hold on Neumora Therapeutics’ schizophrenia therapy Phase 1 trial due to preclinical data showing convulsions in dosed rabbits.
- In April 2024, Reviva Pharmaceuticals announced it has aligned with the FDA on its Phase III program for brilaroxazine in schizophrenia.
- In March 2024, Acadia Pharmaceuticals reported disappointing results from its Phase III ADVANCE-2 trial, where its antipsychotic candidate pimavanserin failed to meet its primary endpoint for controlling negative symptoms in schizophrenia.
Bridging the Gap in the Schizophrenia Market
Decades of research are an eye-witness of the unmistakable contribution of antipsychotics in improving the quality of life and brain functioning; however, the impact of these drugs is different on different patients because antipsychotics have different affinities towards different binding sites present in the brain. Further, these treatments continue for around five years or even much longer after stabilization to avoid the recurrence of the episodes. Since, these work via modulation of dopamine, these are only effective in managing positive symptoms, and leave negative symptoms untended. Also, antipsychotics result in heterogeneous acute effects in the blood oxygen level-dependent (BOLD) signal. Besides, there is a cascade of challenges in the biological interpretation of the neuronal changes in the body, that is still under the shadows. To crown it all, today, approximately 30% of the Schizophrenia patients are resistant to the available antipsychotic drugs. Between 80-90% of the patients experience relapse due to nonadherence to maintenance therapy. This calls for an intensification of R&D in the Schizophrenia market space to better understand the MoA of the drugs, and offer quantitative biomarkers to predict the changing brain activity related to drug action. A need for therapeutic agents potential enough to address cognition impairment, overcome drug-resistance, and treat negative symptoms is strongly felt in the Schizophrenia market.
Thus, to bridge these broadening gaps in the treatment of Schizophrenia, several pharma companies in the Schizophrenia market are investigating novel therapies.

As per DelveInsight, The market size of schizophrenia in the 7MM was approximately USD 8 billion in 2023, which is expected to grow at a significant CAGR by 2034.
Various potential early-phase therapies that are projected to enter the schizophrenia market during the forecast period include (AQW051) Novartis Pharmaceuticals, (JX11502MA) Zhejiang Jingxin Pharmaceutical Co., Ltd., (HS-10380) Jiangsu Hansoh Pharmaceutical Co., Ltd., (NBI-1117568) Neurocrine Biosciences, (SPG302) Spinogenix, (CVL-231) Cerevel Therapeutics, LLC, (LB-102) LB Pharmaceuticals Inc., (MK0557) Merck Sharp & Dohme LLC, (Luvadaxistat) Neurocrine Biosciences, and others.
Iclepertin (BI-425809) is an investigational glycine transporter 1 (GlyT1) inhibitor developed by Boehringer Ingelheim, aimed at addressing cognitive symptoms associated with schizophrenia. By enhancing NMDA receptor function, Iclepertin may improve synaptic plasticity and cognition. Currently undergoing multiple Phase III trials, it also shows potential for Alzheimer’s disease, though its earlier Phase II results for that indication were inconclusive. Ulotaront (SEP-363856), developed by Sumitomo Pharma and Otsuka Pharmaceuticals, is a trace amine-associated receptor 1 (TAAR1) agonist that uniquely does not target dopamine D2 or serotonin 5-HT2A receptors. This small-molecule oral agent is in late-stage development for schizophrenia and generalized anxiety disorder. Despite halting some Phase III trials, two are still ongoing, with a revised licensing agreement giving Otsuka exclusive rights to develop and commercialize Ulotaront worldwide. Both therapies have the potential to reshape the treatment landscape for schizophrenia, offering new hope for patients.
In a nutshell, the schizophrenia market is buzzing with excitement as pharma companies dive into developing pipeline schizophrenia therapies that could change the game. The next decade promises a wave of new treatments, poised to boost global revenue for companies in the schizophrenia space. While recent years have seen a rise in mental health awareness, there’s still a long road ahead in improving long-term treatment outcomes and enhancing patients’ quality of life. Beyond medications, the emotional and social support patients desperately need remains a critical gap.
Schizophrenia demands more than just medicine—it’s about shattering outdated stigmas, debunking myths, and treating mental illness with the same urgency and compassion as physical ailments. Ultimately, it’s not just the pipeline therapies that will reshape the future—it’s the growing awareness and collective mindset shift that will truly ease the global burden of schizophrenia.

Downloads
Article in PDF
Recent Articles
- Hope on the Horizon: Brilaroxazine’s Promise for Schizophrenia Patients
- Breaking Boundaries: Innovations and Updates in Schizophrenia Treatment
- US/AZ deal; Alkermes’ Schizophrenia drug; Innovent/Lilly’s China market expansion; Re...
- Vanda Broadens its Reach to Bipolar Disorder Treatment Market With Fanapt Approval
- How are Antipsychotics Transforming the Schizophrenia Treatment Space?