Creating Future of MedTech Industry with Artificial Intelligence
Dec 07, 2020
Artificial Intelligence is a breakthrough technology and has been used tremendously in this data-driven age, leading to a leap forward in the MedTech industry. Artificial Intelligence enrolls the use of advanced machine learning algorithms and fulfills the job, which has been tackled by humans for ages. It has flourished extensively over the past years owing to its versatility in its applications in the industry.
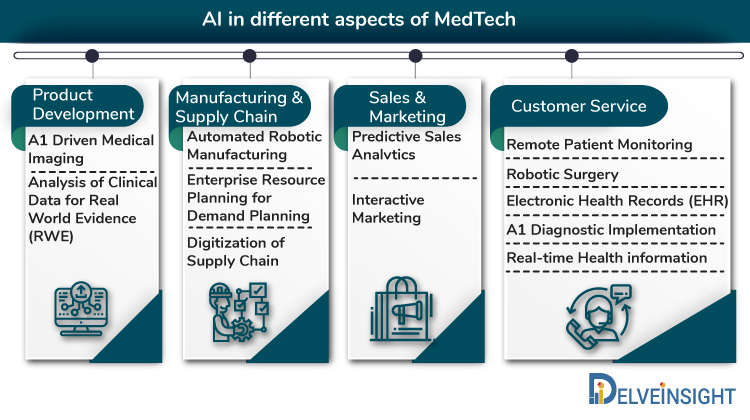
Artificial Intelligence has the hallmarks of becoming a potent tool in the Healthcare industry by improving the current system by identifying unknown variables, solving fundamental issues, and providing viable solutions. For instance, the shortage of healthcare professionals can be tackled by creating better diagnostic and treatment procedures, integrating solutions developed by physicians worldwide, and providing them seamlessly over a platform accessed by all practicing physicians. This aspect can be seen in some clarity via the NAVIFY tumor board developed by Roche for the oncology therapy area. Unlike most other industries, in MedTech, AI is not confined to sales and operations ends of the value chain. Development in the technology has made it possible for solutions to surface, primarily related to R&D, clinical trials, and diagnostics and treatment, as well as patient management, leading to even more significant innovative potential and promise for AI in the industry.
With the introduction of AI in existing business and services models, the revenue models of medical device organizations are also shifting increasingly, including operating expenditure versus their historical focus solely on capital expenditure.
Downloads
Article in PDF
Recent Articles
- Boston Scientific’s WATCHMAN FLX™ Pro; Quest Diagnostics’s AAV Test; Vitestro Started A.D.O.P.T. ...
- Assessing the Emerging Trends in the Evolving Implantable Drug Delivery Device Landscape
- GE HealthCare’s CARESCAPE Canvas Patient Monitoring Platform; Medtronic’s MiniMed™ 780G Sy...
- How Is Digital Therapeutics Reshaping the Future of Healthcare?
- LivaNova Launched SenTiva DUO; Candela Launched Matrix System; 3M Launched Medical Adhesive; MONA...
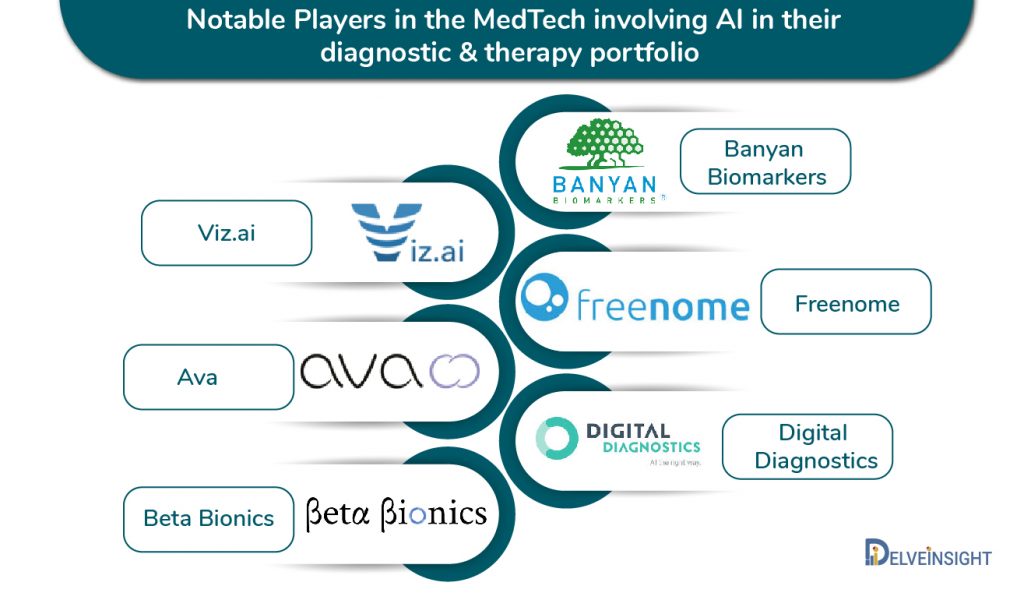
Banyan Biomarkers is one company to be able to market a blood-based diagnostic test in the US market, as it utilizes tech to aid the detection of traumatic brain injuries and concussions. In February 2018, the San Diego-based company was granted a de novo request from FDA for the Banyan Brain Trauma Indicator. The test identifies Ubiquitin and Glial Fibrillary Acidic Protein (GFAP)- 2 brain-specific protein biomarkers- that appear rapidly in the blood of a patient after a brain injury.
Freenome is using AI to develop a colorectal cancer screening application that can learn from its mistakes. In May 2020, Freenome initiated AI-EMERGE, a clinical study for the AI-Genomics blood test has been completed, which collected samples from up to 3,000 patients in the US and Canada.
In April 2018, the FDA approved IDx‘s AI-based diagnostic system for the autonomous detection of diabetic retinopathy, a disorder that can lead to blindness. The IDx-DR solution involves a small robotic camera that takes images of the eye. An AI algorithm then analyses the picture taken from the camera of the patient’s retina and helps decide if a diabetes patient is suffering from retinopathy.
Viz.ai‘s Contact application is a clinical decision support software, designed to analyze CT Scan results, which can notify physicians of a potential stroke in their patients. The application can send a text notification to the specialist if it detects a suspected large vessel blockage through the patient’s scans. The program’s algorithm helps decrease time-lapse as it notifies the specialist during the time of the standard review of images, potentially saving lives.
Women’s health start-up Ava has combined AI and wearable -two of the fastest-growing segments in MedTech – to develop a fertility tracking mobile device. In May 2018, Ava raised about $30 million in a Series B round.
The start-upstart-up is combining AI and CGM to develop a device known as the iLet biharmonic pancreas system. In May 2018, the firm received IDE approval for a trial, and in December 2019, it received the FDA’s Breakthrough Device designation. The iLet mimics a biological pancreas and consists of a dual-chamber, autonomous, infusion pump. The device is worn on the patients’ body, which contains a biharmonic cartridge in it that carries insulin and glucagon. The device can be configured to inject a specific dose of the hormones into the patient. The device is expected to become a game-changer in diabetes and congenital hyperinsulinism disease areas.
Downloads
Article in PDF
Recent Articles
- Airway Management Devices: Charting the Evolving Market Trends and Key Innovations
- 22 Healthcare Trends & Innovations to Watch in 2022 and Beyond
- How Robots Are Introducing A New Dimension To Healthcare Service Delivery
- Ears to the Future: Evaluating Key Advancements and Trends Driving the Audiology Devices Market G...
- MedTech Industry Roars Back as FDA Approvals Soar



