Top 10 Most Promising Drugs In The Amyotrophic Lateral Sclerosis Treatment Landscape
Dec 25, 2020
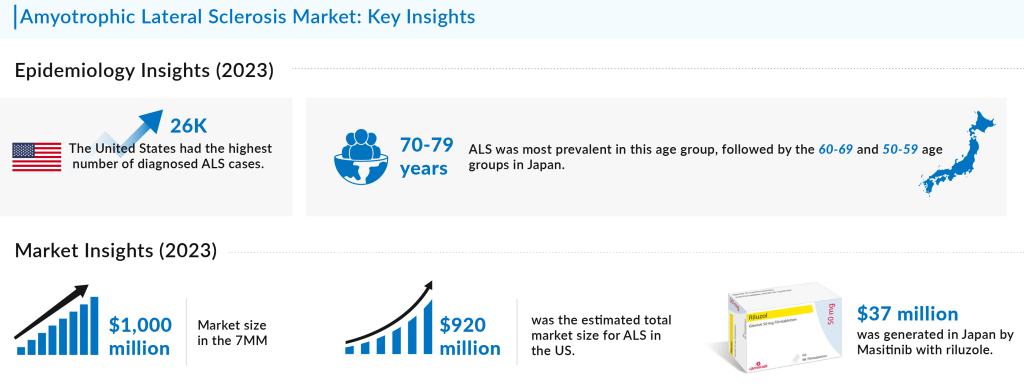
Amyotrophic lateral sclerosis (ALS), commonly known as Lou Gehrig’s disease, is a group of rare neurological diseases that mainly involve the nerve cells (neurons), responsible for controlling voluntary muscle movement. The disease progresses over 3–5 years, making voluntary movements of arms and legs impossible. It is the most common form of motor neuron disease in adults. In 2023, the US reported the highest prevalence of ALS among the 7MM (US, EU4, UK, and Japan), with approximately 26,000 cases, a figure anticipated to rise during the forecast period. Within the EU4 and the UK, Germany had the highest number of diagnosed ALS cases, whereas Spain recorded the lowest. The most common sites of ALS onset were spinal and bulbar, with spinal onset accounting for the highest number of cases, about 15,000, followed by bulbar onset. In Japan, ALS prevalence was notably higher in the 70-79 age group, followed by the 60-69 and 50-59 age groups.
However, the last decade has experienced a substantial increase in the burden of motor neuron disease. Even then, there exists no cure for the disease. The available treatment options help control the symptoms and prevent unnecessary complications.
Therefore, there is a need for curative therapies. Amyotrophic lateral sclerosis pipeline is quite vast with potential competitors including AB Science, Alector, GSK, BrainStorm Cell Therapeutics, Ionis Pharmaceuticals, MediciNova, Denali Therapeutics, AbbVie, Calico Life Sciences, Clene Nanomedicine Biosciences, Seelos Therapeutics, Prilenia Therapeutics, Rapa Therapeutics, NeuroSense Therapeutics, Helixmith, Transposon Therapeutics, Revalesio Corporation, Annexon Biosciences, Corcept Therapeutics, AL-S Pharma, Sanofi, Denali Therapeutics, Orphai Therapeutics and others.
Downloads
Article in PDF
Recent Articles
- FDA’s New Drug Application to ALS Drug; Approval to BeiGene’s Brukinsa; Roche, Temedica Forges Di...
- Precigen’s PRGN-3006; Yescarta Approved as a First CAR T-cell Therapy for R/R LBCL; Biogen ...
- Thrombolytic Sciences’ trial; NurOwn for ALS; Biosimilar receives approval; Trelegy Ellipta for t...
- The Evolving Landscape of Amyotrophic Lateral Sclerosis: A Fatal Disease!
- Unveiling Amyotrophic Lateral Sclerosis (ALS) Treatment Frontiers: Navigating Challenges, Overcom...
- Masitinib: AB Science
Masitinib (AB1010) is an orally administered tyrosine kinase inhibitor. It modulates mast cells and macrophages’ activity – important cells for immunity – by targeting a limited number of kinases without inhibiting, at therapeutic doses, kinases associated with known toxicities. Masitinib distinguishes itself from other ALS developmental drugs by exerting neuroprotection in both central and peripheral nervous systems. Based on its unique mechanism of action, masitinib can be developed in many conditions in oncology, inflammatory diseases, and certain diseases of the central nervous system. AB Science completed a Phase II/III trial and has attained positive results, followed by a green signal from the US FDA on the IND application. However, the drug is under investigation for a Phase III trial in patients with ALS.
In January 2024, AB Science SA announced that the Committee for Medicinal Products for Human Use (CHMP) has proposed that AB Science submit a written response to the List of Outstanding Issues at D195 of the procedure instead of addressing these issues through the oral explanation. AB Science expects an opinion from the CHMP in the second quarter of 2024.
- NurOwn (MSC-NTF cells): Brainstorm Cell Therapeutics
MSC-NTF cells (NurOwn) are autologous bone marrow-derived mesenchymal stem cells (MSC) induced in culture to secrete high levels of neurotrophic factors (NTFs) that support neuronal growth and survival. Thus, MSC-NTF cells combine MSC’s immunomodulatory therapeutic benefits with enhanced neurotrophic factor secretion.
In October 2023, the company announced a strategic realignment to enable accelerated development of NurOwn for the treatment of ALS. This realignment is designed to
1) Support the company’s plans to conduct a double-blind, placebo-controlled Phase IIIb US clinical trial for NurOwn in ALS with an open-label extension.
2) Continue to publish data from NurOwn’s Phase III clinical trial on biomarkers, long-term safety and survival, and the expanded access program, providing transparency around NurOwn data and progressing ALS drug development.
- ABBV-CLS-7262: AbbVie/Calico
ABBV-CLS-7262, currently in Phase II/III trials, targets eIF2B, a key regulator of the integrated stress response (ISR), which becomes activated in people with ALS. By inhibiting ISR in neurons under cellular stress, ABBV-CLS-7262 restores protein synthesis and dissolves pre-formed TDP-43-containing stress granules. This is clinically significant because TDP-43 inclusions, which arise from these granules, are a hallmark of ALS pathology. The HEALEY ALS Platform trial, initiated in April 2024 by the Sean M. Healey & AMG Center for ALS at Massachusetts General Hospital in collaboration with NEALS, is expediting the testing of multiple ALS therapies. ABBV-CLS-7262 is administered orally, with a mechanism of action classified as a eukaryotic-initiation-factor-2b-stimulant.
- RAPA 501: Rapa Therapeutics
RAPA-501, currently in Phase II/III trials, is an autologous cell therapy designed to mitigate ALS progression by reducing inflammation. This treatment involves reprogramming a patient’s own T-cells to exhibit TREG/Th2 anti-inflammatory properties and a T-stem phenotype, allowing for effective therapy without prior chemotherapy. The mechanism of action focuses on replacing T lymphocytes to combat inflammation and protect motor neurons. In ongoing trials, RAPA-501 has shown safety with no adverse events, biological activity with significant anti-inflammatory effects, and potential in slowing pulmonary function decline. The Phase II/III study involves administering the therapy intravenously to 41 participants, who will receive four injections over 18 weeks, with monitoring for 30 weeks and a follow-up after two years, aiming to establish the highest safe dose and assess its therapeutic impact on ALS and similar diseases.
- AIT-101: AI Therapeutics
AIT-101, a pioneering and highly selective inhibitor of the lipid kinase PIKfyve, represents a novel approach in ALS treatment. By inhibiting PIKfyve, AIT-101 activates the transcription factor TFEB, which enhances the clearance of toxic protein aggregates through the autophagy-lysosomal pathway. This mechanism has shown promise in preclinical studies, demonstrating reduced protein aggregates, improved survival of motor neurons in various ALS models, and amelioration of functional deficits in a mutant TDP-43 animal model. Currently in Phase II trials, AIT-101 has been effective in crossing the blood-brain barrier (BBB) when administered orally and intrathecally, leading to the clearance of abnormal protein aggregates in neural tissues. OrphAI Therapeutics has initiated a clinical trial of AIT-101 (LAM-002A) specifically targeting ALS patients with a C9orf72 mutation (C9ALS), focusing on evaluating its safety, tolerability, BBB penetration, and effects on disease biomarkers and functional outcomes. This initial trial aims to measure the reduction of C9 repeat expansion proteins, with future studies expected to expand to a broader ALS patient population.
- MN-166: MediciNova
The MN-166 (ibudilast) portfolio, featuring its lead drug compound and proprietary analogs, stands out as a pioneering, first-in-class, non-opioid therapeutic approach for drug addiction, progressive multiple sclerosis, and pain management. MN-166 is an orally bioavailable, small-molecule glial attenuator that targets pro-inflammatory cytokines such as IL-1ß, TNF-a, and IL-6 while potentially boosting the anti-inflammatory cytokine IL-10. Additionally, it acts as a functional antagonist of toll-like receptor 4 (TLR4), contributing to its neuroinflammatory attenuation. Although MN-166 is considered a New Molecular Entity (NME) in the United States and Europe, it is based on ibudilast, a drug with over 20 years of clinical use in Japan, where it has been prescribed to over 3.2 million patients with a solid post-marketing safety record.
On September 9, 2024, MediciNova, Inc., announced that the COMBAT-ALS study of MN-166 (ibudilast) will be featured in a poster presentation at the 35th International Symposium on ALS/MND, scheduled for December 6-8, 2024, in Montreal, Canada. The presentation will be delivered by Dr. Björn Oskarsson, MD, FAAN, Associate Professor of Neurology at Jacksonville, FL, and Director of the ALS Center of Excellence. MN-166 is currently advancing through Phase II/III clinical trials for ALS, continuing to show promise in its role as a novel treatment for this challenging neurodegenerative disease.
- RNS60: Revalesio
RNS60 is an emerging therapeutic agent being studied for various neurological conditions, including amyotrophic lateral sclerosis, Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, and traumatic brain injury. Although its precise mechanism of action is still under investigation, RNS60 is known for its immunomodulatory and cytoprotective properties, demonstrated in animal models of neuroinflammation and neurodegeneration. A Phase II study published in the European Journal of Neurology highlighted RNS60’s potential benefits for ALS patients, showing a slower rate of decline in forced vital capacity (FVC) compared to placebo and significant improvements in the eating and drinking domain of the ALS Assessment Questionnaire-40. This study also noted that neurofilament light chain levels remained stable in patients treated with RNS60, whereas they increased in those receiving placebo.
Currently in Phase II trials, RNS60 is designed to aid recovery from neurological trauma, such as ischemic stroke, and to address chronic neurodegenerative diseases linked to mitochondrial dysfunction, like ALS. The drug activates intracellular signaling pathways that reduce inflammation, promote mitochondrial biogenesis, and enhance cell survival. It protects neurons and oligodendrocytes while modulating immune responses to restore cellular balance, with fewer side effects than many existing treatments. RNS60 has earned FDA Orphan Drug and Fast Track designations for ALS and has shown promising preclinical and Phase I clinical results. It can be administered either through inhalation (nebulization) or intravenous infusion.
- VM202: Helixmith
VM202 is an innovative gene therapy designed to promote tissue regeneration by injecting hepatocyte growth factor (HGF) directly into peripheral nerves. This therapy aims to protect nerves, support neuron growth, and counteract atrophic conditions. VM202 has received FDA Orphan Drug and Fast Track designations and is currently in Phase II clinical trials for ALS. The therapy employs a nonviral delivery system using a plasmid to introduce the HGF gene into nerve cells, which helps promote angiogenesis, prevent muscle shrinkage, and enhance nerve cell survival. Regular injections are needed to maintain HGF production as the genetic material does not integrate into the patient’s DNA.
Engensis, another key player in ALS treatment, involves intramuscular injections of a plasmid encoding two forms of HGF. This gene therapy is designed to boost HGF levels, which may slow ALS progression by enhancing nerve and muscle regeneration. In early trials, Engensis was well-tolerated and showed some promising trends in stabilizing muscle strength and function. The ongoing Phase IIa REViVALS-1A study aims to further assess its safety and tolerability in a larger cohort, with results expected to provide deeper insights into its potential benefits for ALS patients.
- Dazucorilant: Corcept Therapeutics
Dazucorilant is a selective cortisol modulator developed by Corcept Therapeutics, targeting the glucocorticoid receptor (GR) without interacting with other hormone receptors. This drug, which can cross the blood-brain barrier, is under investigation for its potential in treating ALS and other neurological disorders. Dazucorilant is protected by a suite of patents, including those for its composition and methods of use.
The DAZALS trial is a Phase II, randomized, double-blind, placebo-controlled study involving 198 patients with ALS. The trial is designed to evaluate the efficacy and safety of dazucorilant in ALS patients. As of April 15, 2024, enrollment in the Phase II DAZALS trial was completed across multiple countries, including Belgium, France, Canada, Ireland, the UK, Germany, Poland, Spain, the Netherlands, and the USA. Additionally, Corcept initiated a Phase I drug-drug interaction trial in healthy volunteers on May 31, 2024 (NCT06495944), and no recent updates have been reported for Phase I trials in the UK.
- QRL-201: QurAlis Corporation
QRL-201 is a pioneering therapeutic candidate designed to restore STMN2 expression in ALS patients. STMN2 is crucial for neural repair and axonal stability, but its expression is markedly reduced in nearly all ALS cases. QRL-201 has shown promise in reversing STMN2 loss of function in motor neuron disease models derived from ALS patients, particularly those with TDP-43 pathology. Currently in Phase I clinical trials, QRL-201 is being tested in a double-blind, multiple-ascending dose study involving approximately 64 ALS patients. Participants are assigned to receive QRL-201 or a placebo in a 6:2 ratio, with the trial featuring six dose escalation cohorts and two exploratory cohorts. The study aims to collect extensive biomarker data, includes sentinel dosing, and involves rigorous safety reviews, marking QRL-201 as a groundbreaking approach to restoring critical protein function in ALS.
Conclusion
In the relentless fight against ALS, the horizon is brightening with a promising array of innovative therapies that offer hope where there once was only uncertainty. From Masitinib’s unique neuroprotective strategies to NurOwn’s cutting-edge stem cell therapy, and ABBV-CLS-7262’s breakthrough in stress response modulation, the landscape is evolving rapidly. RAPA 501‘s autologous approach, AIT-101‘s novel mechanism targeting protein aggregates, and MN-166‘s dual-action anti-inflammatory properties are shaping up to be game changers. Meanwhile, RNS60‘s broad-spectrum neuroprotection and VM202‘s regenerative gene therapy further underscore a new era of personalized and potent treatments. Dazucorilant and QRL-201 bring their own revolutionary approaches, each with the potential to transform the lives of ALS patients.
As we stand on the cusp of these exciting advancements, it’s clear that the future of ALS treatment is not just a dream—it’s rapidly becoming a reality. With every trial, every breakthrough, and every hopeful study, we are edging closer to a world where ALS is no longer an insurmountable challenge but a manageable condition. The journey ahead may still be long, but with these groundbreaking therapies lighting the way, the promise of a better tomorrow for ALS patients shines ever brighter.

Downloads
Article in PDF
Recent Articles
- Merck’s Keytruda Wins Another FDA Approval; Sanofi Pauses Trial of Myasthenia Gravis Drug, tolebr...
- Genentech’s gantenerumab Fails in Phase III Trial; CHMP Recommends’ Dupixent; FDA Clears Imfinzi ...
- The Evolving Landscape of Amyotrophic Lateral Sclerosis: A Fatal Disease!
- Thrombolytic Sciences’ trial; NurOwn for ALS; Biosimilar receives approval; Trelegy Ellipta for t...
- Denali’s Impressive Research Portfolio