Unmet Needs and Emerging Trends in the Acute Respiratory Distress Syndrome Market Landscape
Nov 20, 2024
Acute Respiratory Distress Syndrome (ARDS) is a severe lung condition characterized by acute respiratory distress syndrome symptoms such as rapid onset of noncardiogenic pulmonary edema and hypoxemia. This life-threatening condition is a leading cause of respiratory failure, particularly in infants and critically ill patients. ARDS is triggered by a range of factors, including pulmonary causes like pneumonia and aspiration, and non-pulmonary causes such as sepsis, pancreatitis, and trauma. These conditions contribute to the development of non-hydrostatic pulmonary edema, which significantly impairs lung function.
In terms of acute respiratory distress syndrome treatment, the focus is primarily on addressing the underlying co-morbidities and providing supportive care. Mechanical ventilation remains the cornerstone of ARDS treatment, with protective ventilation being a key approach during the acute phase to minimize further lung injury. The progression of systemic and pulmonary inflammation during the first week of mechanical ventilation plays a critical role in determining the patient’s outcome—whether the condition resolves or persists.
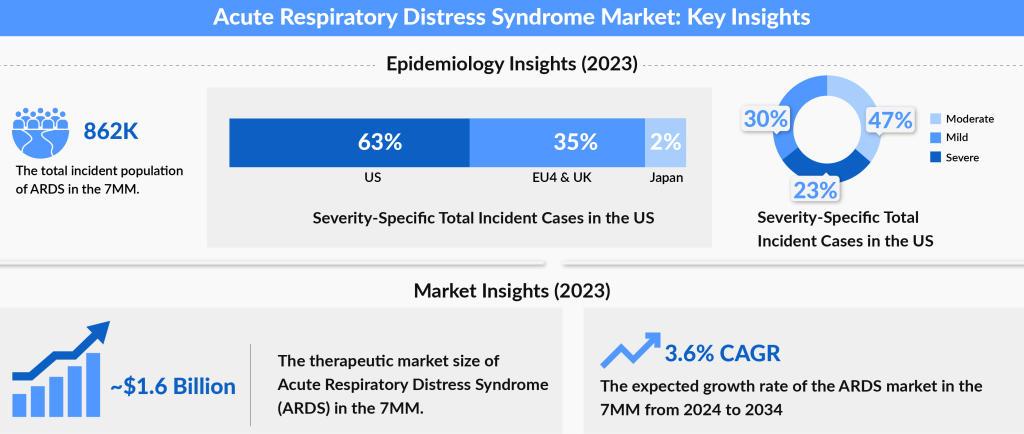
The increasing prevalence of ARDS and its associated complications has led to significant interest in the acute respiratory distress syndrome market. With ongoing advancements in ARDS treatment options and the development of emerging therapies, there is a growing opportunity for innovation in both management strategies and potential breakthroughs in improving patient outcomes.
Downloads
Article in PDF
Recent Articles
- Supply shortage: Why ventilators are in demand amid the Coronavirus crisis
- Rising Acute Respiratory Distress Syndrome (ARDS) Prevalence Posing a Major Public Health Concern
- In the fight against COVID-19 – Lilly with Olumiant, Gilead with remdesivir, Rutgers with testing...
- Alnylam’s lumasiran results; AZ’s oncology drug; Astellas Roxadustat; Evotec’s partnership with ABL
- Watch Out for the Most Promising Therapies in the Pipeline in ARDS Market
To fight lung infection, which is pretty much commonly observed among ARDS patients, Antibiotics are given. Glucocorticoids as end-effectors of the hypothalamic-pituitary-adrenal axis are the most important physiologic inhibitors of inflammation, affecting hundreds of genes involved in stress-related homeostasis.
Several experiments have observed that β2 agonists can help accelerate alveolar epithelial repair, injury to which is a considerable cause of ARDS. β2 agonists can increase sodium transport by activating β2 receptors on alveolar type I and type II cells, accelerating the resolution of pulmonary edema. Furthermore, experimental and human studies also vote in favor of Keratinocyte growth factor (KGF) considering it an important for alveolar epithelial repair.
Statins—a class of drugs that is often prescribed by doctors to help decrease cholesterol levels in the blood— can be used to address inflammation, an additional pathological hallmark of ARDS. However, some studies have shown the results quite the opposite, heralding them as non-beneficial neither in lowering the morbidity of ARDS in high-risk patients nor in improving the clinical outcomes of ALI/ARDS patients.
According to various studies, neuromuscular blocking agents (NMBAs) induce reversible muscle paralysis and, thus are important in the management of a large number of hospital patients. Pancuronium and Vecuronium are the most commonly used NMBAs for the management of ARDS. Although the use of NMBAs in ARDS treatment is not marginal, it remains highly debatable owing to inherent risks in patients in the ICU.
Other treatment options that patients with ARDS are generally prescribed include supplemental oxygen, prone positioning, paralytic use, fluid management, and a technique called positive end-expiratory pressure (PEEP) to release excess fluid out of air sacs. These are given in combination with continuing treatment of the original illness or injury. Also, acute respiratory distress syndrome supportive therapy, such as intravenous fluid or food, may be needed.
However, the treatment is supportive in nature. Despite decades of research, treatment options for ARDS are restricted. Only a few pharmacological therapies have emerged for ARDS. Supportive care with mechanical ventilation remains the mainstay of management. In the off-label treatment options, STATIN therapy, NMBAs, and glucocorticosteroids are recommended because there is no approved therapy for this indication yet. There are relatively few treatments available for ARDS and mortality remains high at 35 to 45% in most studies due to organ failure, sepsis, age, and associated comorbid illness.
Furthermore, unrecognized ventilator disconnections can quickly lead to hypoxemia and hypercarbia and cause cardiopulmonary collapse.Inadequate sedation and analgesia in a paralyzed patient can cause extreme psychologic distress. Besides confinements with treatments, ARDS diagnosis has always remained challenging owing to incomplete knowledge of the syndrome. Most ARDS cases are not diagnosed at any time during a patient’s stay in the intensive care unit (ICU) leading to a delayed diagnosis.
Thus, there is a need for potential therapeutic advancements in the Acute Respiratory Distress Syndrome Market to provide treatments that are curative in nature. Studies show that premium-price agents such as stem cell therapies and other pipeline candidates with a better clinical profile are soon to grace the ARDS market. Several pharmaceutical companies are investigating novel therapeutic agents strengthening the pipeline.
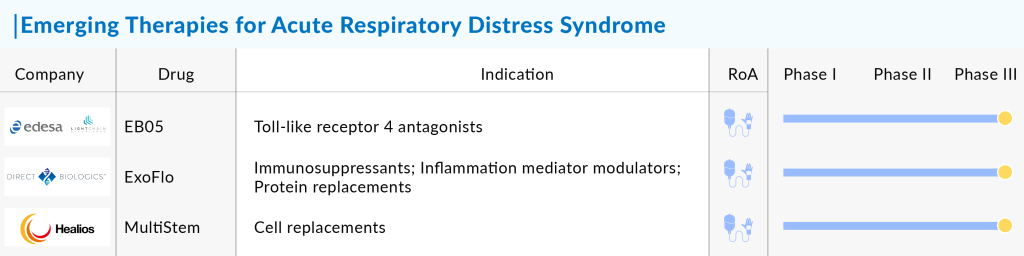
Key companies such as Biomarck Pharmaceuticals, Athersys, Healios, Direct Biologics, Biohaven Pharmaceutical, Arch Biopartners, APEPTICO Forschung und Entwicklung GmbH, Staidson (Beijing) Biopharmaceuticals, MediciNova, Edesa Biotech, Light Chain Biosciences, Boehringer Ingelheim, Genentech, Windtree Therapeutics, Veru, Mesoblast Limited, Avalo Therapeutics, Pluristem Therapeutics, ILTOO Pharma, and others are proactively engaged in propelling Acute Respiratory Distress Syndrome Market Size creating a positive impact on the market.
The Acute Respiratory Distress Syndrome therapeutic market in the 7MM is projected to grow at a CAGR of 3.6%, driven by increasing disease awareness, improved diagnostics, and the introduction of emerging therapies. In 2023, the market size was estimated at approximately USD 1,643 million. The expected launch of emerging ARDS pipeline therapies, such as Sabizabulin, ExoFlo, MultiStem, Ibudilast, and and others shall transform the ARDS market landscape. The market, which is dominated by supportive therapies soon will embrace innovative therapies that shall expedite the steady growth throughout the study period (2020 to 2034).
The growth of the Acute Respiratory Distress Syndrome (ARDS) market is expected to be driven by the entry of upcoming pipeline therapies, rising ARDS incidence, and increased awareness of the disease. With advancements in ARDS treatment and significant investments in research and development, particularly in response to the global COVID-19 pandemic, a promising environment has emerged to accelerate the adoption of new acute respiratory distress syndrome supportive therapies. This dynamic landscape presents substantial opportunities for pharmaceutical companies to explore and capture market share in the evolving ARDS treatment space, offering hope for improved patient outcomes and a better clinical profile for emerging therapies.

Downloads
Article in PDF
Recent Articles
- In the fight against COVID-19 – Lilly with Olumiant, Gilead with remdesivir, Rutgers with testing...
- Supply shortage: Why ventilators are in demand amid the Coronavirus crisis
- Rising Acute Respiratory Distress Syndrome (ARDS) Prevalence Posing a Major Public Health Concern
- Alnylam’s lumasiran results; AZ’s oncology drug; Astellas Roxadustat; Evotec’s partnership with ABL
- Watch Out for the Most Promising Therapies in the Pipeline in ARDS Market