Pertussis Vaccines: Whole-cell & Acellular Vaccines, Different Types & Manufacturers along with the Regimen in Various Age Groups
Dec 20, 2021
Table of Contents
Pertussis, also commonly known as whooping cough, is a very highly contagious respiratory disease. It is caused by the bacterium Bordetella Pertussis. Pertussis can be a serious disease for people of all ages but especially for babies. Pertussis vaccines offer the best protection against this very contagious disease. One way to ensure the prevention of Pertussis is staying up to date with Pertussis vaccines. Pertussis can lead to violent and uncontrollable coughing which often makes it hard to breathe. After cough fits, someone with Pertussis often needs to take deep breaths, which results in a “whooping” sound. Pertussis can affect people of all ages but can be very serious, even deadly, for babies less than a year old.
Pertussis Vaccine
Pertussis vaccine protects against whooping cough (Pertussis). There are two main types of Pertussis vaccine: whole-cell vaccines and acellular vaccines. The whole-cell vaccine is observed to be 78% effective while the acellular vaccine is 71–85% effective. The effectiveness of the vaccines appears to decrease by between 2 and 10% per year after vaccination with a more rapid decrease with the acellular vaccines. Acellular Pertussis vaccines are more commonly used in the developed world due to fewer adverse effects. Between 10 and 50% of people given the whole-cell vaccines develop redness at the injection site or fever. Febrile seizures and long periods of crying occur in less than 1% of people. Acellular Pertussis vaccines were developed after studies raised concerns about a temporal relation between Pertussis-containing vaccines and neurologic illnesses, including encephalopathy, infantile spasms, and sudden infant death syndrome. With the Acellular Pertussis vaccines, a brief period of non-serious swelling of the arm may occur. Side effects with both types of vaccines, but especially the whole-cell vaccine, are less common the younger the child. The whole-cell vaccines should not be used after 7 years of age. Serious long-term neurological problems are not associated with either type.
Downloads
Article in PDF

WHO/CDC and Pertussis Vaccine
The World Health Organization (WHO) and the Center for Disease Control and Prevention (CDC) recommend all children be vaccinated for Pertussis and that it be included in routine vaccinations. Additional doses may be given to older children and adults. This recommendation includes people who have HIV/AIDS. The Pertussis vaccine was developed in 1926. It is on the World Health Organization’s List of Essential Medicines. The prevention of Pertussis centers on community vaccination. Recent immunization efforts have focused on adults because they are the primary transmitters of the disease to infants and are at risk of infection themselves. The whole-cell Pertussis vaccine in combination with diphtheria and tetanus toxoids was introduced in 1948.
Two vaccines in the United States help prevent whooping cough: DTaP and Tdap. These vaccines also provide protection against tetanus and diphtheria. Children younger than 7 years old get DTaP, while older children, teens, and adults get Tdap. DTP, DTaP and TDaP vaccines are used for the prophylactic treatment of Pertussis. Many vaccines are available in the market for different age groups. Adacel and Boostrix are available for adults and pregnant women. Post-exposure Pertussis treatment is also available in the market through different antibodies like macrolides (erythromycin, clarithromycin, or azithromycin) and Trimethoprim-sulfamethoxazole.
Types of Pertussis Vaccines
Following is the list of most commonly used Pertussis Vaccine manufactured by some of the leading pharma companies in the Pertussis landscape –
DTP (Sanofi Pasteur)
A series of four doses of whole-cell DTP vaccine was found to be 70–90% effective in preventing major Pertussis disease in controlled efficacy trials done in the 1940s and subsequent observational efficacy studies. Up to 50% of whole-cell DTP vaccination doses resulted in local responses such as redness, swelling, and discomfort at the injection site.
Quattrovac (Astellas)
It is a DPT vaccine with a bulk of inactivated poliomyelitis vaccine derived from purified attenuated polioviruses (Sabin type 1, 2, and 3) cultured in Vero cells (a cell line derived from African green monkey kidney cells) made by the Japan Poliomyelitis Research Institute (JPRI). Marketing authorization was approved for the first time in Japan in July 2012.
DTaP or DT Vaccination (Serum Institute of India)
It should be given to children under the age of seven (in instances where the Pertussis vaccine component is contraindicated or where the physician decides that the Pertussis vaccine is not to be administered). Acellular Pertussis vaccines vary concerning the manufacturer, the number of components, quantity of each component, methods of purification and toxin inactivation, and incorporation adjuvants and excipients.
Pentacel (Sanofi Pasteur)
For children aged 6 weeks to 4 years, the DTaP-IPV/Hib vaccine is authorized as a four-dose series. Given to infants between the ages of two and four, six, and fifteen to eighteen months. The first three doses must be spaced out by at least four weeks. Doses 4 and 3 must be separated by at least 6 months, and Dose 4 should not be given before 12 months.
DTaP – Infanrix and Daptacel (Sanofi Pasteur)
For children aged 6 weeks to 6 years, the DTaP (diphtheria, tetanus toxoids, and acellular Pertussis vaccination) is recommended. A primary series of three doses are given at ages 2, 4, and 6 months, followed by a booster dosage between ages 15 and 18 months and another booster dose between ages 4 and 6 years (total of 5 doses). The first three doses should be taken every 4–8 weeks. Dose 4 should be given at least 6 months after Dose 3 and not before the age of 12 months. Doses 4 and 5 of both DTaP brands are advised for children aged 15–18 months (15 through 20 months for Daptacel). If at least 6 months have passed after Dose 3 and, in the opinion of the vaccine provider, the child is unlikely to return for another visit between the ages of 15 and 18, Dose 4 may be given as early as 12 months.
Pediarix (GlaxoSmithKline)
The DTaP-HepB-IPV vaccine is authorized for use in children aged 6 weeks to 6 years in a three-dose series. It is given to newborns at the ages of 2, 4, and 6 months. The DTaP component determines the minimum intervals for the DTaP-HepB-IPV vaccine. The three dosages must be separated by at least four weeks. The DTaP-HepB-IPV vaccination cannot be used for the birth dose of the hepatitis B (HepB) vaccine since the minimum age for the first dose is 6 weeks.

Vaxelis (Merck & Co; Sanofi Pasteur)
For children aged 6 weeks to 4 years, DTaP-IPV-Hib-HepB is recommended as a three-dose regimen. It is given to newborns at the ages of 2, 4, and 6 months. The DTaP component determines the minimum intervals for the DTaP-IPV-Hib-HepB vaccine. The three dosages must be separated by at least four weeks. The DTaP-IPV-Hib-HepB vaccination cannot be used for the birth dose of the hepatitis B (HepB) vaccine since the minimum age for the first dose is 6 weeks.
Kinrix (GlaxoSmithKline)
The DTaP-IPV (Kinrix) vaccine is exclusively licensed for Dose 5 of the DTaP vaccination and dosage 4 of the IPV vaccine in children aged 4–6 years who have previously had their DTaP vaccine doses using Infanrix and/or Pediarix for doses 1, 2, and 3, and Infanrix for Dose 4. The dose Kinrix vaccine does not need to be repeated if it is given to children who have previously received another brand of DTaP vaccine for past DTaP vaccine doses.
Quadracel (Sanofi Pasteur)
Only Dose 5 of the DTaP vaccination and dosage 4 or 5 of the IPV vaccine are approved for children aged 4–6 years who have received four doses of Pentacel and/or Daptacel vaccine. The dosage of DTaP-IPV (Quadracel) vaccine does not need to be repeated if it is given to children who have previously received another brand of DTaP vaccine for past DTaP vaccination doses, or if it is given as Dose 1, 2, 3, or 4 of the DTaP vaccine series or Dose 1, 2, or 3 of the IPV series.
HexyonTM/Hexacima (Sanofi Pasteur)
DTaP-IPV-Hib-HepB vaccine for primary and booster vaccination of infants from six weeks of age. It is the only fully liquid, ready-to-use, 6-in-1 vaccine to protect infants against diphtheria, tetanus, Pertussis (whooping cough), Hepatitis B, poliomyelitis, and invasive infections caused by Haemophilus influenzae type b. The use of acP (acellular Pertussis) antigens and IPV (inactivated poliovirus vaccine) improves safety and reduces reactogenicity as compared to wcP (whole-cell Pertussis)-containing vaccines and OPV (oral polio vaccine).
Tetrabik (Biken Co. Ltd)
DPT-sIPV vaccine is an adsorbed diphtheria-purified Pertussis-tetanus-inactivated polio (Sabin-strain) combined vaccine. A suspension for injection in 0.5-mL single-dose prefilled syringes. Each 0.5 mL dose contains ≥4 units of the Bordetella Pertussis protective antigen, ≤15 Lf of diphtheria toxoid, ≤2.5 Lf of tetanus toxoid, 1.5 DU of inactivated poliovirus type 1 (Sabin strain), 50 DU of inactivated poliovirus type 2 (Sabin strain), and 50 DU of inactivated poliovirus type 3 (Sabin strain) as active ingredients.
Tribik (BIKEN; Mitsubishi Tanabe Pharma Corporation)
It is an adsorbed diphtheria-purified Pertussis-tetanus combined vaccine. A 0.5 mL dose of Tribik (Diphtheria and Tetanus toxoids and acellular Pertussis vaccine) vaccine is approved for administration to infants and children 6 weeks to 7 years of age as a five-dose series. The series consists of a primary immunization course of three doses administered at 2, 4, and 6 months of age, followed by two booster doses, recommended at 15–18 months of age, and at 4–6 years of age, respectively.
TDaP (Sanofi Pasteur)
Tdap vaccine can prevent tetanus, diphtheria, and Pertussis. Diphtheria and Pertussis spread from person to person. Tetanus enters the body through cuts or wounds. Tdap is only for children 7 years and older, adolescents, and adults. Tdap may be given at the same time as other vaccines.
Boostrix and Adacel (Sanofi Pasteur)
The FDA has approved both Tdap vaccines as a booster dose for anyone who has already received the standard pediatric DTP/DTaP vaccination series. Boostrix is only for people over the age of ten. Adacel is only licensed for a single dose in people aged 10–64. A second dose of Adacel is also approved for use for tetanus prophylaxis when indicated for wound management if at least 5 years have passed since the previous receipt of any tetanus toxoid-containing vaccine and for use for tetanus prophylaxis when indicated for wound management if at least 5 years have passed since the previous receipt of any tetanus toxoid-containing vaccine.
Post-exposure Treatment of Pertussis
Antibiotics may prevent Pertussis disease if given prior to symptom onset, but there are no data to indicate that widespread use of Pertussis and Postexposure Antimicrobial Prophylaxis (PEP) among contacts effectively controls or limits the scope of Pertussis outbreaks. Macrolide antibiotics (e.g., erythromycin, clarithromycin, or azithromycin) have been effective and constitute the mainstay of treatment for patients with Pertussis as well as for PEP. Trimethoprim-sulfamethoxazole (also known as co-trimoxazole) is used as an alternative to Macrolides.
Pertussis Vaccine Regimen in Different Age Groups
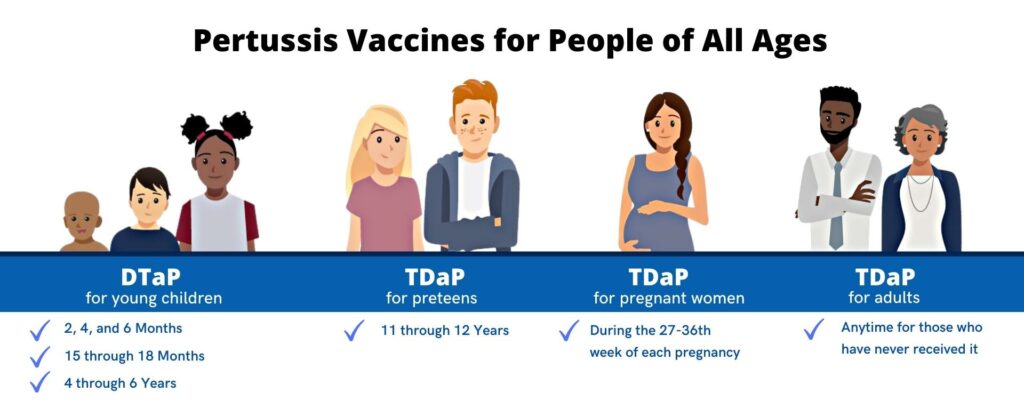
Babies need 3 shots of DTaP to build up high levels of protection against diphtheria, tetanus, and whooping cough. Then, young children need 2 booster shots to maintain that protection through early childhood. Shots at the following ages are recommended – 2 months, 4 months, 6 months, 15 through 18 months, 4 through 6 years. For children who should not get Pertussis vaccines, healthcare professionals can give DT instead of DTaP. For example, children who had a very bad reaction to DTaP can receive DT. However, children who get DT will not receive any protection against whooping cough. Preteens and Teens should get one shot of Tdap between the ages of 11 and 12 years to boost their immunity. Teens who didn’t get Tdap as a preteen should get one shot the next time they visit their healthcare professional. Pregnant Women should get Tdap during the early part of the 3rd trimester of every pregnancy. By doing so, she helps protect her baby from whooping cough in the first few months of life. Vaccinating the mother during pregnancy may protect the baby. Three doses starting at six weeks of age are typically recommended in young children. Adults who have never even once received the Pertussis vaccine should get a shot of Tdap. This can be given at any time and should be followed by either a Td or Tdap shot every 10 years.
What Lies Ahead For the Pertussis Vaccine
The Food and Drug Administration (FDA) licensed many combination vaccines for use in order to help protect against Pertussis. These vaccines help protect a lot against Pertussis/whooping cough. Among the approved vaccines for Pertussis, FDA has only approved DTaP including Daptacel, Infanrix, Kinrix, Pediarix, Pentacel, Quadracel, Vaxelis, and TDaP including Adacel and Boostrix while Repevax/Adacel-Polio and Hexaxim are approved only in Europe. On the other hand, Quattrovac, Tetrabik, and tribik are approved in Japan whereas Pentaxim is only approved in China.
There is an increase observed in Pertussis cases observed in the last decade. Although not life-threatening (excluding infants), due to the availability of so many vaccines, Pertussis was a potentially fatal illness in yesteryears. Expected launch of potential Pertussis vaccines BPZE1, BK1310/ MT-2355, SIIPL Tdap, DTcP Infant and Booster, DTacP, and others, may increase Pertussis market size in the coming years, assisted by an increase in vaccination coverage of Pertussis.

FAQs
Most of the people getting the Pertussis vaccine haven’t experienced severe side effects or serious problems with it. However, a few side effects may occur and they are usually mild, not affecting any daily activity.
Diphtheria, Tetanus, and Pertussis vaccination are highly recommended for everyone.
Pertussis is a respiratory illness, also commonly known as whooping cough. It is a highly contagious disease caused by a type of bacteria called Bordetella pertussis.
Protection decreases over time, so adults need to get a Td or Tdap booster shot every 10 years to stay protected.
Downloads
Article in PDF
Recent Articles
- Notizia
- Actinium Announces SIERRA Trial Results; Santhera Seeks FDA Review for Vamorolone; Seres Announce...
- Merck’s Sotatercept Trial Result; PARP Rivals Closing in on AstraZeneca and Merck’s Lynparza; FDA...
- Vir’s drug shows results; Abbott launches coronavirus antibody lab test; Reprogrammed skin ...
- Global Hepatitis B Market Size



