Pompe Disease: Causes, Symptoms, Diagnosis, Pathology, Current Marketed and Emerging Drugs in the Pipeline
Sep 30, 2024
Table of Contents
Pompe disease is an inherited disorder caused by the buildup of a complex sugar called glycogen in the body’s cells. The accumulation of glycogen in certain organs and tissues, especially muscles, impairs their ability to function normally. There are three types of Pompe disease, which differ in severity and the age at which they appear. These types are known as classic infantile-onset, non-classic infantile-onset, and late-onset Pompe disease.
Pompe disease is caused due to mutations in the GAA gene. The GAA gene provides instructions for producing an enzyme called acid alpha-glucosidase (also known as acid maltase). This enzyme is active in lysosomes which are structures that serve as recycling centers within cells. The enzyme normally breaks down glycogen into a simpler sugar called glucose, which is the main energy source for most cells. Mutations in the GAA gene prevent acid alpha-glucosidase from breaking down glycogen effectively, which allows this sugar to build up to toxic levels in lysosomes. This buildup damages organs and tissues throughout the body, particularly the muscles, leading to the progressive signs and symptoms of Pompe disease. Pompe disease is inherited in an autosomal recessive pattern which means both copies of the gene in each cell have mutations. The parents of an individual with an autosomal recessive condition each carry one copy of the mutated gene, but they typically do not show signs and symptoms of the condition. There are many different and more scientific terms for Pompe Disease including Acid maltase deficiency, Alpha-1,4-glucosidase deficiency, GAA deficiency, Glycogenosis type II, and a few others.
Downloads
Article in PDF
Recent Articles
- Advances in Pompe Disease Treatment: From ERT to New Therapies
- Top 12 Most Expensive Drugs in the US Healthcare Market
- 5 Promising Pompe Disease Drugs in Early-stage Development
- Assessment of Key Products that Got FDA Approval in Second Half (H2) of 2021
- Navigating Challenges in Pompe Disease Treatment and Research in Eastern Europe
Pompe disease symptoms begin in the first months of life, with feeding problems, poor weight gain, muscle weakness, floppiness, and head lag. Respiratory difficulties are often complicated by lung infections. The heart is grossly enlarged. Many infants with Pompe disease also have enlarged tongues. Pompe disease affects about 1 in 40,000 people in the United States. The incidence of this disorder varies among different ethnic groups. Patients with the ‘classic infantile’ form of Pompe disease are the most severely affected. Although hardly any symptoms may be apparent at birth, the disease usually presents within the first three months of life with rapidly progressive muscle weakness (‘floppy infants’), diminished muscle tone (hypotonia), respiratory deficiency, and a type of heart disease known as hypertrophic cardiomyopathy, resulting in diminished cardiac function. These problems together culminate in cardio-respiratory failure within the first 2 years of life. Many infants have a large, protruding, tongue and moderate enlargement of the liver. The legs often rest in a frog position and feel firm on palpation.
The diagnosis of Pompe disease is based on a thorough clinical evaluation, a detailed patient and family history, and a variety of biochemical tests with first of all the measuring of GAA activity. In individuals suspected of having Pompe disease, blood can be drawn and the function/activity of GAA (the ‘enzymatic activity’) can be measured in white blood cells (leukocytes), but only if the proper assay conditions are being used and acarbose is added to the reaction mixture to inhibit the activity of glucoamylase. Alternatively, the GAA activity/functional assay can also be performed on dried blood spots, but this method is not any quicker, less reliable, and also requires the use of acarbose to inhibit the glucoamylase activity. A variety of other tests can be performed to detect or assess symptoms potentially associated with Pompe disease such as sleep studies, tests that measure lung function, and tests that measure muscle function. Muscle MRI (imaging by magnetic resonance) is used to visualize the degree of muscle damage.
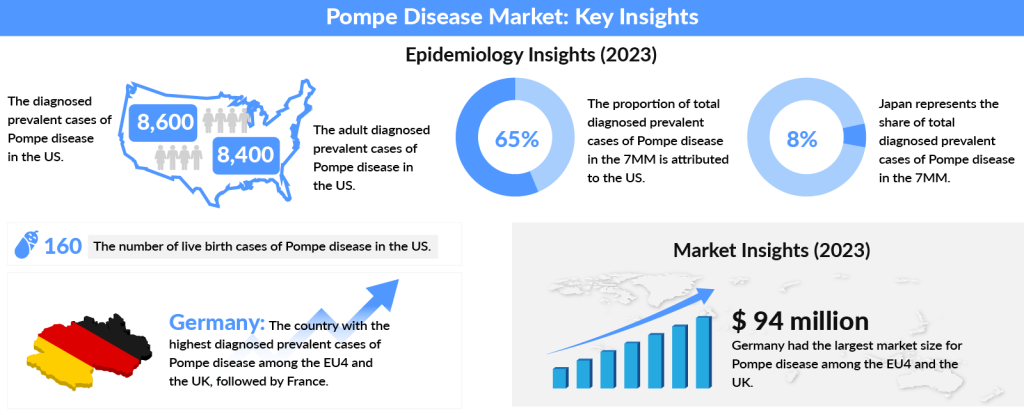
As per DelveInsight, the US accounted for nearly 65% of the total diagnosed prevalent cases of Pompe disease across the 7MM in 2023, with the number expected to increase by 2034. In 2023, the diagnosed prevalent cases of Pompe disease in the US were approximately 8,600, with adult cases making up around 8,400. Among the EU4 and the UK, Germany had the highest number of diagnosed prevalent cases, followed by France. Japan, on the other hand, accounted for about 8% of the total diagnosed prevalent cases of Pompe disease within the 7MM in 2023, while Spain had the least diagnosed cases.
Many key pharmaceuticals are proactively involved in developing Pompe Disease treatment therapies including companies like Immusoft, Asklepios Biopharmaceutical, Oxyrane, Maze Therapeutics, Amicus Therapeutics, Spark Therapeutics, and many others
Currently, there is no permanent cure for Pompe disease. But different treatment options can help ease Pompe disease symptoms. Most patients require supportive therapy to address the symptoms which include respiratory and cardiac problems, physical disability, and difficulty swallowing. Some patients may require a mechanical ventilator during respiratory infections or at night. Physical and occupational therapy can improve patients’ muscle strength and help them to learn to use canes or walkers if necessary. Speech therapy and the usage of feeding tubes are some of the other components.
Pompe Disease Current Treatment Landscape
Pompe disease treatment is disease-specific, symptomatic, and supportive. Treatment requires the coordinated efforts of a team of specialists with expertise in treating neuromuscular disorders. Pediatricians or internists, neurologists, orthopedists, cardiologists, dieticians, and other healthcare professionals may need to systematically and comprehensively plan an affected child’s treatment.
Enzyme Replacement Therapy (ERT) is a cornerstone in the Pompe disease treatment landscape. It involves the intravenous administration of recombinant human acid α-glucosidase. This therapy aims to boost the levels of GAA in the body and mitigate the excessive accumulation of glycogen inside cells. The enzyme, produced in the laboratory through genetically modified cultured cells, is purified and then introduced into the bloodstream. ERT helps alleviate the abnormal thickening of heart walls, a hallmark of Pompe disease, and supports muscle function, ultimately enhancing patients’ quality of life.
The advent of Pompe disease therapy through ERT has revolutionized the management of this condition, significantly altering its natural history, extending patient lifespan, and improving morbidity. Despite these advancements, results have not fully met expectations, and many patients still face challenges associated with the disease. Thus, treatment for Pompe disease often includes symptomatic and supportive measures. These may involve physical therapy to bolster strength, respiratory support via mechanical ventilation (such as BiPAP or volume ventilators) during respiratory infections, and other interventions like occupational therapy, which might include canes or walkers. Additionally, speech therapy can aid in improving articulation and communication for some patients. The ongoing support and comprehensive care are crucial for managing Pompe disease effectively and enhancing Pompe disease life expectancy with treatment.
In April 2006, under priority review, the FDA approved a Biologic License Application for the orphan drug Myozyme (alglucosidase alfa, recombinant human [rh] GAA) for patients with Pompe disease.
Being the only FDA-approved curative treatment option for the disease it occupied a major market share despite its inherent adverse reactions including pneumonia, respiratory failure and distress, infections, and fever. This was the first disease-specific treatment for an inherited muscular disorder consisting of the intravenous administration of recombinant human GAA (Myozyme/Lumizyme). Prior to the arrival of Enzyme replacement therapy (ERT) in 2006, patients were primarily dependent on Supportive Treatment comprising of respiratory support, ambulatory support, physiotherapy and/or dietary treatment. Although historically, Supportive Care had been the mainstay of treatment (thereby generating a huge amount of revenue), but the approval and subsequent launch of ERT in 2006 after decades of research, helped turn the treatment landscape upside down, prompting a new era in the treatment of this disease.
Although enzyme replacement therapy (ERT) is not a cure, it has played a critical role in slowing or halting the progression of Pompe disease. The introduction of ERT has marked a major therapeutic advancement, significantly benefiting patients by preventing further disease progression, despite its high cost. This has positioned ERT as a pivotal treatment option in managing Pompe disease.
However, the landscape is evolving, and recent advances in the Pompe disease market are expanding beyond ERT to include exciting new therapies. These emerging treatments, such as gene therapies and next-generation drugs, hold the potential to revolutionize patient care further, offering new hope for better management and possibly long-term solutions for those affected by Pompe disease. The shift from reliance on ERT to these new therapies reflects a broader transformation in the approach to treating Pompe disease.
On August 06, 2021, the FDA approved NEXVIAZYME (avalglucosidase alfa-ngpt) for intravenous infusion to treat patients 1 year of age and older with late-onset Pompe disease to Genzyme Corporation. NEXVIAZYME, another Enzyme Replacement Therapy, is an intravenous medication that helps reduce glycogen accumulation. The effectiveness of NEXVIAZYME for the treatment of Pompe disease was demonstrated in a study of 100 patients who were randomized to take NEXVIAZYME or another FDA-approved enzyme replacement therapy for Pompe disease. The most common side effects included headache, fatigue, diarrhea, nausea, joint pain, dizziness, muscle pain, itching, vomiting, difficulty breathing, skin redness. In 2022, NEXVIAZYME was approved in Europe. By February 2024, new data indicated that treatment with NEXVIAZYME significantly improved ptosis, or drooping eyelid, in pediatric patients with infantile-onset Pompe disease (IOPD) over nearly three years.
The currently approved rhGAA can significantly improve Pompe disease’s therapeutic effect, in order to provide improved delivery of rhGAA to the lysosome, new technologies have been explored. Small molecule pharmacologic chaperones are currently being evaluated as therapeutic options in several single-gene disorders, including lysosomal storage disorders, where the pathology can be attributed to a misfolded but still functional enzyme, albeit, with reduced activity.
In September 2023, the FDA approved POMBILITI (cipaglucosidase alfa-atga) + OPFOLDA™ (miglustat), marking a significant advancement in Pompe disease treatment. This groundbreaking two-component therapy combines POMBILITI™, a bis-M6P-enriched recombinant human acid α-glucosidase (rhGAA) designed for high-affinity uptake through the M6P receptor while maintaining its potency, with OPFOLDA, an oral enzyme stabilizer that reduces the loss of enzyme activity in the bloodstream.
POMBILITI and OPFOLDA are specifically indicated for adults with late-onset Pompe disease (LOPD) who weigh 40 kg or more and have not achieved improvement with their current ERT. This new therapy represents a promising option for enhancing Pompe disease management and improving patient outcomes. In February 2024, the 20th Annual WORLDSymposium, awarded Amicus Therapeutics’ POMBILITI + OPFOLDA the 2024 New Treatment Award.

Pompe Disease Emerging Drugs
AT-GAA developed by Amicus Therapeutics is an investigational therapy that consists of ATB200, a unique recombinant human acid alpha-glucosidase (rhGAA) enzyme with optimized carbohydrate structures, particularly mannose-6 phosphate (M6P), to enhance uptake, co-administered with AT2221, a pharmacological chaperone. In Feb 2019, the US Food and Drug Administration (FDA) granted Amicus a Breakthrough Therapy Designation to AT-GAA for the treatment of late-onset Pompe disease. In February 2023, Amicus Therapeutics announced positive results from the global Phase 3 open-label extension (OLE) study (ATB200-07), which is currently ongoing. This study assessed the long-term efficacy and safety of AT-GAA in adults with late-onset Pompe disease. The results showed that participants treated with AT-GAA for up to 104 weeks exhibited persistent and durable improvements in six-minute walk distance (6MWD), stability in forced vital capacity (FVC), and continued reductions in biomarkers of muscle damage and disease substrate.
Recombinant Adeno-Associated Virus Acid Alpha-Glucosidase is being developed by Lacerta Therapeutics, Inc. and is currently in the Phase I stage of development.
Another Pompe disease gene therapy ACTUS-101 currently being developed by Actus Therapeutics is delivered into the blood, specifically targeting the liver to promote the production of the GAA enzyme. However, it is in an early stage of development and recently dosed the first patient for the Phase I/II trial. It would be too soon to predict the impact it will have on the market if and once approved.
VAL-1221 being developed by Valerion Therapeutics is a recombinant fusion protein comprised of rhGAA and a lupus anti-DNA antibody 3E10 with GAA (acid glucosidase), the missing enzyme in GSD Type II (that is believed to utilize the nucleoside transporter ENT2 for cellular uptake of ERT in an M6P-independent manner). The drug candidate is currently in Phase I/II stage of clinical development by Valerion Therapeutics for the treatment of late-onset Pompe disease.
AT845 by Audentes Therapeutics (now a part of Astellas) is a novel Pompe disease product candidate expected to address the recognized limitations of ERT by facilitating the intracellular production of GAA to improve efficacy, reduce immunogenicity, and deliver a durable and transformative clinical benefit from a single intravenous administration. Currently, AT845 is in Phase I/II clinical trials for LOPD. In February 2023, Astellas announced preliminary results from the Phase I/II first-in-human clinical trial, FORTIS, which assessed the safety, tolerability, and exploratory efficacy of AT845 in adults with LOPD. These results were presented at the 19th Annual WORLDSymposium in Orlando.
GC301, developed by Beijing GeneCradle Pharmaceutical, is another promising drug in the pipeline for infantile-onset Pompe disease (IOPD). This gene therapy utilizes a recombinant adeno-associated virus (AAV) to deliver the GAA gene expression sequence systemically. The therapy has received notification of drug clinical trial approval from the Center for Drug Evaluation, National Medical Products Administration (CDE-NMPA), and has been approved by the clinical trial ethics committee of Peking Union Medical College Hospital (PUMCH). GC301 is currently in Phase I/II clinical trials, but the studies are exclusively conducted in China.
The biotech companies Shire and NanoMedSyn launched a new research partnership to evaluate a potential treatment for lysosomal storage disorders including Pompe disease.
| Company Name | Emerging Drugs | Clinical Trial Phase |
| Amicus Therapeutics | AT-GAA | Phase I/II |
| GeneCradle Pharmaceutical | GC301 | Phase I/II |
| Actus Therapeutic | ACTUS-101 | Phase I/II |
| Audentes Therapeutics | AT845 | Phase I/II |
| Valerion Therapeutics | VAL-1221 | Phase I/II |
What is in Store for Pompe Disease?
There are many key pharmaceutical players in the Pompe Disease therapeutic market landscape such as Sanofi, Actus, Amicus, Roche, and others which are anticipated to develop novel and potential treatment therapies which will enhance treatment outcomes in Pompe patients. In addition to that, investigational gene therapies have the potential to provide a long-term, steady supply of GAA enzymes to the entire body through one-time administration. These kinds of new and innovative researches provide hope for a bright future for the treatment of Pompe Disease.
Also, recently the FDA has been granting many drugs Priority Review, Fast Track & Breakthrough Therapy designations which may provide incentives to assist and encourage the development of drugs for rare diseases.

FAQs
Life expectancy for late-onset Pompe disease is currently estimated to be age 30 when it first appears in children or teenagers, and 50 years of age for adults. But the infantile form of Pompe disease is extremely serious and usually fatal with a life expectancy of less than 2 years.
Unfortunately, there is no cure for Pompe disease. However, Pompe disease has benefited from the introduction of enzyme replacement therapy (ERT), which, although expensive, is a major therapeutic advance.
The disease is rare. In the United States, only 1 person in 40,000 is affected by Pompe disease. It can affect both males and females of all ethnic groups.
Downloads
Article in PDF
Recent Articles
- Avrobio taps Magenta’s ADC; Eli Lilly ramps up; FDA postpones decision; CSL gears up to fight pan...
- Rising of Orphan Drug Development
- Navigating Challenges in Pompe Disease Treatment and Research in Eastern Europe
- The Transforming Pompe Disease Treatment Landscape in Emerging Markets
- Top 12 Most Expensive Drugs in the US Healthcare Market