What are Monoclonal Antibodies and How Can They Treat COVID-19?
Jan 03, 2022
Table of Contents
An antibody is a protein produced by the immune system in response to an infection in the body. Whereas, a monoclonal antibody is a molecule produced in a laboratory specifically designed to enhance the body’s natural immune system response against an invader, such as cancer or an infection. Monoclonal antibodies have a certain type of advantage over other types of treatment for infection because they are generated specifically to target an essential part of the infectious process. A Monoclonal antibody is created by exposing a white blood cell to a particular viral protein, furthermore, it is cloned to produce a large number of antibodies to target that virus such as COVID-19.
Monoclonal antibodies are not chemical compounds like most drugs are, they are the cloned replica of natural antibodies, so they are those proteins only that the body produces to defend itself against a disease. The exception being they are laboratory-created and mass-produced which is why they are also at times known as ‘designer antibodies’. They are customized, tailor-made, specifically designed to treat a certain kind of disease.
Downloads
Article in PDF
Recent Articles
- Comprehensive Assessment of the Leading Companies in the Vaccines Market
- Algernon’s NP-120; FDA nod to 4DMedical tool; SaNOtize’s NORSTM trial; Pole’s c...
- Evaluating the Upcoming Drugs in Pipeline for Major Autoimmune Diseases
- Keeping a track on COVID-19 through Wearable Technology
- Actinium Announces SIERRA Trial Results; Santhera Seeks FDA Review for Vamorolone; Seres Announce...
The first Monoclonal antibody therapy was licensed more than 30 years ago. Since then, millions of people have been benefitted continuously from more than 100 such treatments. Around 50 of these Monoclonal antibody therapies were brought to market in the past six years alone. It is considered as one of the fastest-growing fields in biomedical research, and also a very important segment of the pharmaceutical market. Most of the best-selling drugs in the past year were Monoclonal antibodies. They were developed to treat several viral infections, such as Ebola, rabies and treat life-threatening diseases such as cancer.
As it is known, antibodies are naturally produced in the body to fight off infections, but when the body is introduced to an extremely novel virus such as COVID-19, it does not have antibodies specifically present for the disease to fight it off. And this is where the role of Monoclonal antibodies comes into action. Due to the fact that Monoclonal antibodies are created in a laboratory specifically designed to target a particular virus or infection such as COVID-19. They are considered to be one of the most efficient treatments of COVID-19 patients who are at a high risk of progression to severe disease.
Mechanism of Action for Monoclonal Antibody Therapy
Antibodies are produced naturally in response to a foreign protein/antigen and are one of the ways that the body defends itself against a disease. Antibodies specifically bind to their particular targets, like viruses, bacteria or cancerous cells. They block the action of their target protein making them harmless, or they flag it as a foreign particle so that other parts of the immune system can clear the ‘invaders’ away. Monoclonal antibodies operate in the same manner as well. They bind specifically to their specific target, without harming anything else in their way. Due to their numerous applications, Monoclonal antibodies have been safely and effectively used to treat a growing number of diseases, some of which were difficult to treat in the past such as the COVID-19.
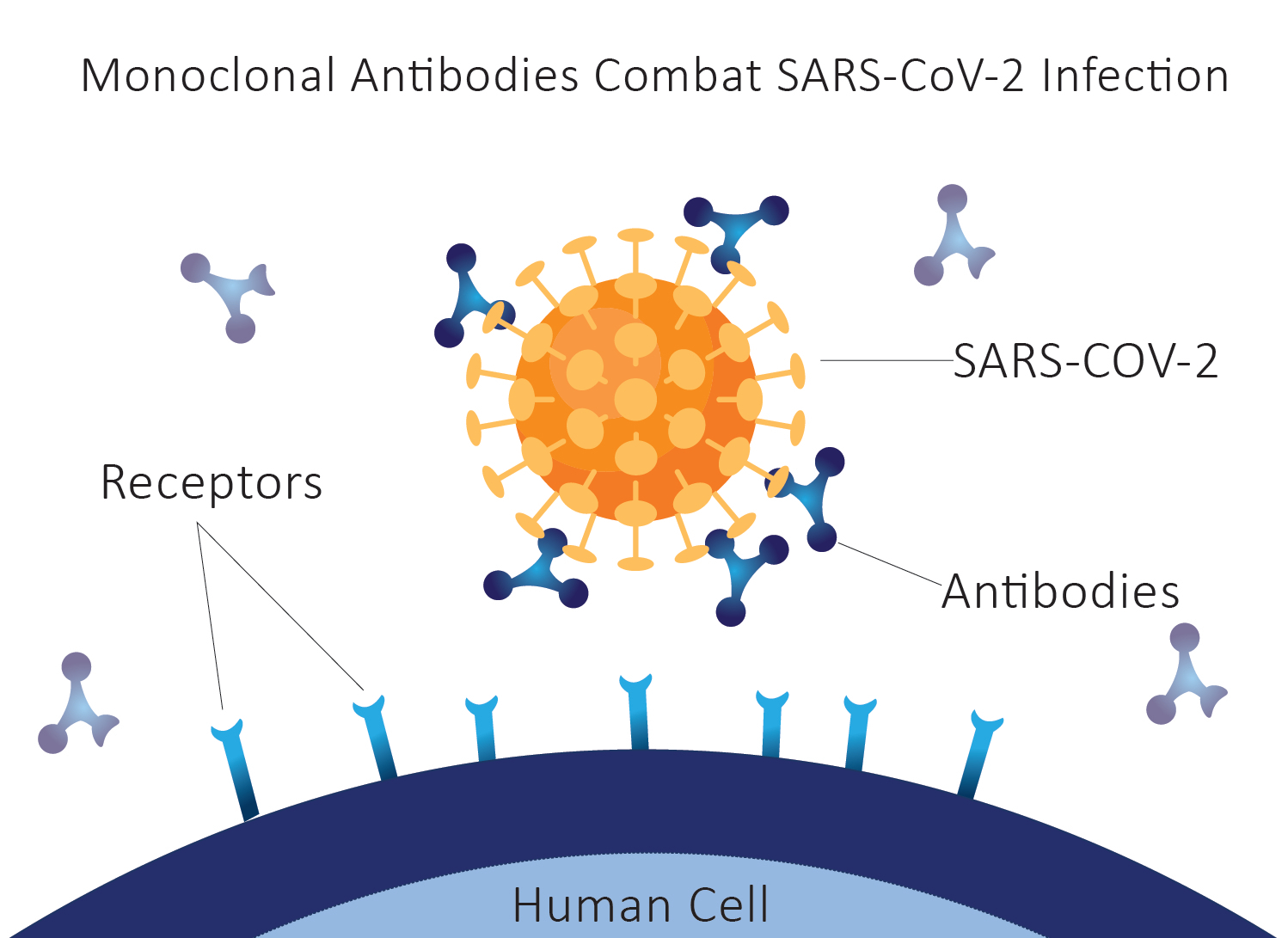
Monoclonal Antibody Treatment for COVID-19
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)/COVID-19 has the appearance of a spike protein on its surface that helps the virus to attach and enter human cells. Several Monoclonal antibodies have been developed to bind to that particular spike protein of SARS-CoV-2 and block the virus from invading human cells. Patients with COVID-19 can sometimes receive an intravenous (IV) infusion of Monoclonal antibodies, usually in an emergency department, an infusion center, or another outpatient setting (such as the patient’s home or nursing home).
A variety of new variants of the SARS-CoV-2 virus have recently been detected. These variants emerge because of mutations in the genome of the virus. Monoclonal antibodies remain effective against the new SARS-CoV-2 variant. However, some mutations may cause changes in the spike protein that could interfere with the effectiveness of currently available Monoclonal antibodies.
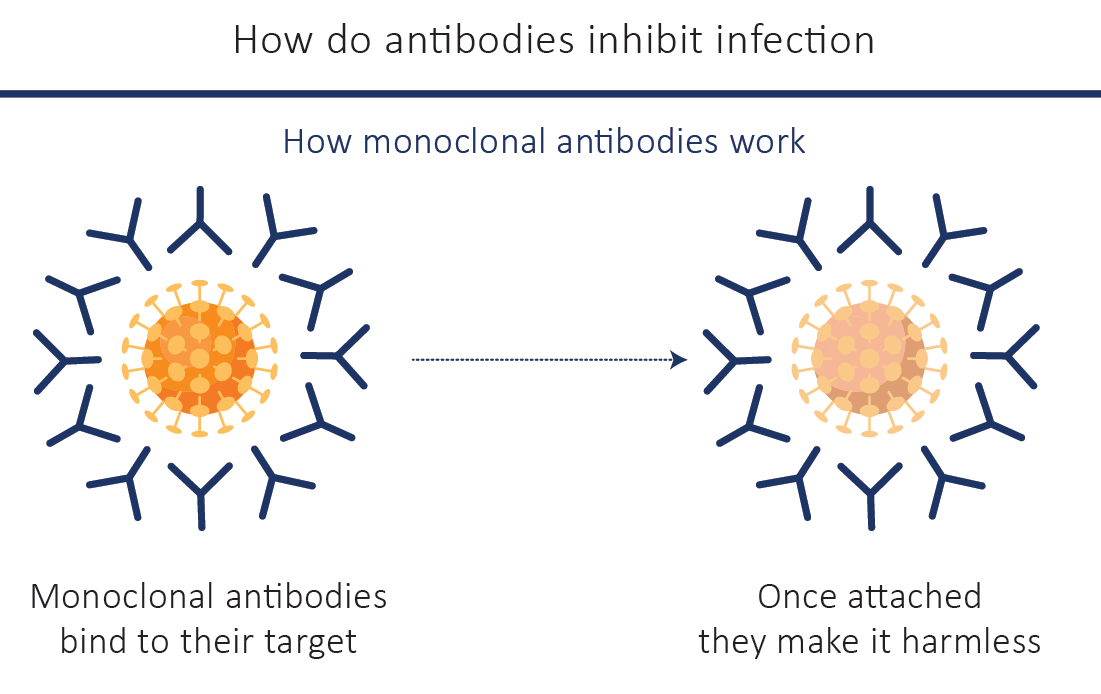
FDA Approved Monoclonal Antibody Therapy for COVID-19
The FDA has authorized the following mentioned investigational Monoclonal antibody therapy under emergency use authorization (EUA) for pre-exposure prophylaxis of COVID-19:
Tixagevimab co-packaged with cilgavimab (EUA issued December 8, 2021), to be administered as 2 separate consecutive intramuscular injections.
Casirivimab and imdevimab, to be administered together (EUA issued November 21, 2021).
Bamlanivimab and etesevimab, to be administered together (EUA issued February 9, 2021).
Sotrovimab (EUA issued May 26, 2021).
Tocilizumab (EUA issued June 24, 2021).
The above-mentioned Monoclonal antibody therapies are FDA approved to treat mild-to-moderate COVID-19 in adults and pediatric patients when the patient has a positive COVID-19 test result and the patient is also at high risk for progressing to severe COVID-19 and hospitalization as well. Health care providers may administer Monoclonal antibody therapy only in settings where they specifically have immediate access to medications to treat a severe infusion reaction, such as anaphylaxis, and also the ability to activate the emergency medical system (EMS).
Can Monoclonal Antibodies Treat COVID-19?
For the past 30 years, Monoclonal antibody therapy have transformed the way to treat several immensely fatal diseases, time and then they are proven to be more effective, better tolerated, and easier to deliver in comparison to some other treatments. Researchers are also very optimistic that there is a possibility of Monoclonal antibodies could help prevent and treat early infections of COVID-19.
Few of the advantages offered by Monoclonal antibodies are:
Monoclonal antibodies have the ability to specifically target the SARS-CoV-2 virus, as they originate in the blood of people who have recovered from Covid-19, potentially making them more effective than some other drugs.
They can be rapidly isolated and manufactured, it is recorded that more than 70 monoclonal antibody therapy for COVID-19 are under development, since the initiation of the pandemic.
They have the potential to treat infected patients or prevent infection in all individuals, including the elderly and young children, and immunocompromised people (some who can’t even receive vaccination) as well.
They provide instant protection against infection – once administered into the body, Monoclonal antibodies enter the bloodstream straight away and offer immediate protection for a few weeks or months.
Whereas, on the other hand, vaccines might take a few weeks to have an effect but usually provide long-term protection. This is the sole reason why researchers are optimistic that Monoclonal antibodies could be complementary to vaccines in order to help to contain the pandemic.
Development and Availability of Monoclonal Antibodies for COVID-19
Since the start of the pandemic, researchers have been evaluating existing drugs and developing new treatments which include a lot of Monoclonal antibodies to treat Covid-19 patients. Several monoclonal antibodies that are licensed or in development for other diseases are in clinical trials to see if they have an effect on Covid-19 patients. One of these is adalimumab, which was also used to treat arthritis previously.
The pharmaceutical company Eli Lilly, in collaboration with AbCellera, launched the first human study of potential COVID-19 monoclonal antibody treatment. Other safety clinical trials include studies by AstraZeneca, Regeneron, Celltrion, AvantGen, IGM Biosciences, Vir Biosciences, GSK, and many others. The speed of research put into COVID-19 treatments has been unprecedented, pharma giants such as Eli Lilly and Regeneron published early results, in less than four months since the start of their clinical trials for COVID-19 Monoclonal antibody product showing encouraging signs.
The Monoclonal antibody treatment’s safety and efficacy is one thing, but providing them to huge masses, making them easily available for the patients in need is another task on its own. It requires manufacturing Monoclonal antibodies in large quantities, and also the fact that they are very expensive. Monoclonal antibodies are more complex and expensive to produce than other types of drugs. This makes them some of the most expensive drugs in the world.
Biosimilar products are one way to reduce production costs and make these innovative treatments cheaper. Another way companies can make monoclonal antibodies cheaper is by introducing second brands which can only be sold in low- and middle-income countries.
Future of Monoclonal Antibodies as a Treatment Therapy
Millions of people around the world could benefit from existing Monoclonal antibody treatments and those in development – including the ones for COVID-19, which could help bring the pandemic to an end. These innovative products are not currently accessible for most of the world’s population. That could change if governments, the biopharmaceutical industry, global health organizations and fund providers work together.
Several steps such as spreading the word about how valuable monoclonal antibody therapy can be for treating a range of diseases, developing guidelines for monoclonal antibodies to make sure that these products are designed with the needs of local populations in mind, investment in innovative technologies, new business models for cost-cutting can be taken to make monoclonal antibodies more available across the world.

FAQs
People who receive monoclonal antibody treatment may experience pain at the injection or infusion site, including skin bruising, soreness, swelling, possible infection.
Cost is definitely an issue when it comes to Monoclonal antibodies as they are very expensive.
A vaccine offers protection against the disease, prior to its occurrence, whereas monoclonal antibody treatment is for patients already been tested positive for COVID-19.
Downloads
Article in PDF
Recent Articles
- Keeping a track on COVID-19 through Wearable Technology
- Boston Scientific’s MODULAR CRM System; Restore Medical’s ContraBand clinical trial r...
- J&J collaborates with BARDA; Virus kills pancreatic cancer cells; ALX raises USD 105 million...
- Merck, Dewpoint’s HIV Pipeline; Pfizer, BioNTech ‘s COVID-19 Vaccines; Roche’s Tecent...
- In the fight against COVID-19 – Lilly with Olumiant, Gilead with remdesivir, Rutgers with testing...