Most Promising Oncological Drugs Expected to Launch in 2022
Jan 14, 2022
Table of Contents
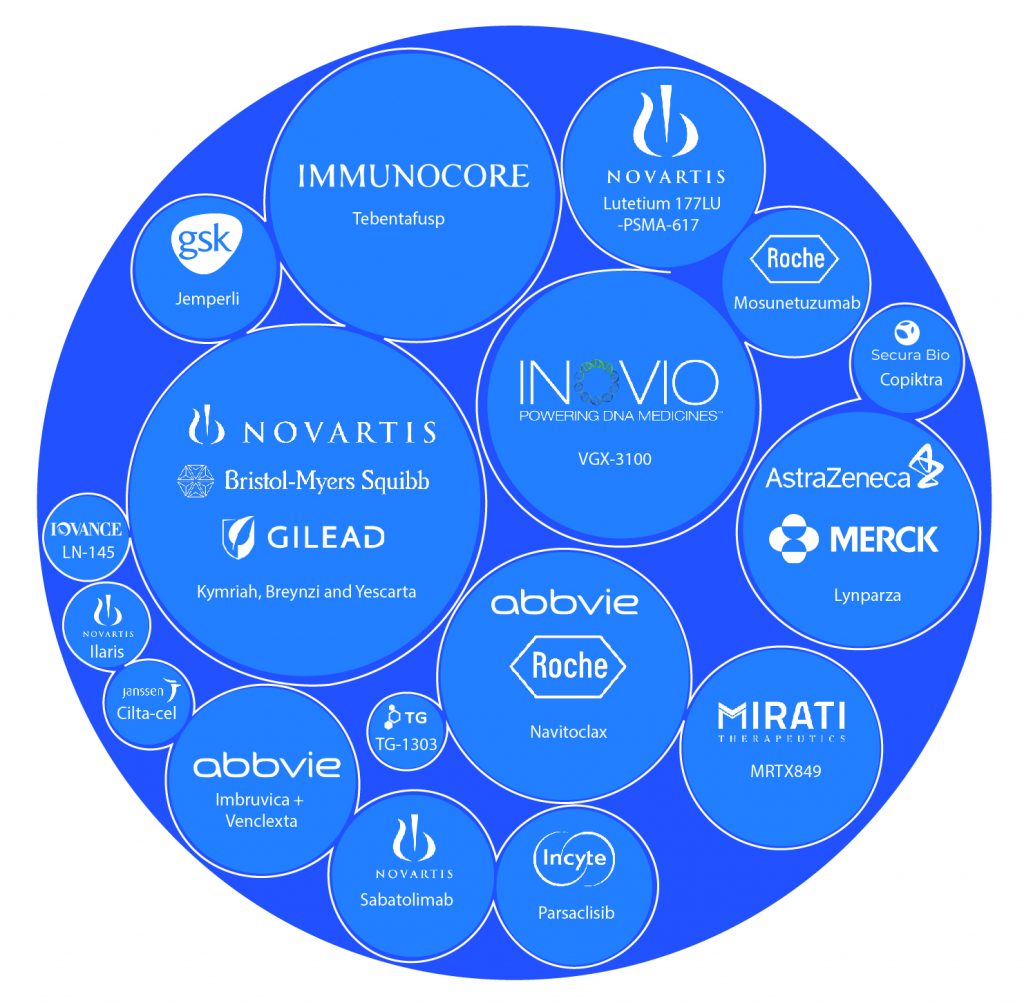
The innovation in the oncology drug pipeline has resulted in a record number of FDA and EU approvals in recent years, as investigators and sponsors seek new and targeted treatments for individuals diagnosed with different types of cancers each year. In 2022, regulators will continue to evaluate new oncology therapies and also the ones approved for other indications for unmet needs in the growing, aging cancer community. The oncology drug pipeline consists of some highly anticipated therapies, including Imbruvica + Venclexta, Kymriah, Yescarta, Navitoclax, and many more. These oncology drugs represent new drug classes, significant changes in clinical practice, and significant market opportunities across a wide range of indications. Let’s dive deep into the upcoming oncology drugs and learn about them.
LN-145: Iovance Biotherapeutics: Cervical Cancer
Drug Name: LN-145
Downloads
Article in PDF
Recent Articles
- Changing Landscape of EBV-associated Diseases Treatment: Is T-cell Immunotherapy the Answer?
- 8 Emerging Bispecific Antibodies Transforming NSCLC Treatment
- Diverse Pipeline Therapies to Glide the Follicular Lymphoma Treatment Market
- 66 Year Ohio Woman Becomes Cancer Free, Thanks to CAR T Cell Therapy
- Amgen’s IMDELLTRA FDA Approval; J&J’s Proteologix Acquisition; Bristol Myers Squibb’s B...
Company: Iovance Biotherapeutics
MOA: Immunologic cytotoxicity; T lymphocyte replacements
Originator: National Cancer Institute (USA); National Institutes of Health (USA)
Special Status: Breakthrough and Fast Track Designation in the US for Cervical Carcinoma (2019)
LN-145 by Iovance Biotherapeutics is an adoptive cell transfer therapy that uses an autologous TIL manufacturing process developed by the NCI for Recurrent, Metastatic, or Persistent Cervical Carcinoma treatment. Patients in this trial were given an NMA lymphocyte-depleting preparative regimen, then an infusion of autologous TIL, then an IL-2 regimen.
In the United States, LN-145 has received Breakthrough and Fast Track designations in 2019, which will expedite a potential approval. LN-145 has shown promising results in recurrent, metastatic, or persistent cervical cancer patients. It could hit the market in 2022, filling an unmet need for effective regimens in a difficult-to-treat population.
The high efficacy of LN-145 should outweigh any concerns about tolerability. LN-145 demonstrated an impressive 11% CRR, 44% ORR, and 85% DCR in pretreated, advanced cervical cancer patients with few treatment options in the innovaTIL-04 study. Patients also responded quickly to treatment, with a mean time to best response of 2.4 months. After 7.4 months, the response time had not been reached. Tolerability, however, may limit its potential, as 96.3 % of patients experienced at least one grade 3/4 adverse event, with relatively high rates of grade 3/4 hematologic side effects.
VGX-3100: Inovio Pharmaceuticals: Cervical Dysplasia
Drug Name: VGX-3100
Company: Inovio Pharmaceuticals
MOA: Gene transference; Immunostimulants
Originator: University of Pennsylvania
Special Status: None
VGX-3100 is DNA-based immunotherapy developed by Inovio Pharmaceuticals as an alternative to surgery and ablation for Cervical High-Grade Squamous Intraepithelial Lesion (HSIL) to preserve reproductive health while treating precancerous disease.
Currently, Inovio’s Phase 3 Cervical HSIL program is evaluating the efficacy of VGX-3100 in regressing Cervical HSIL, a direct precursor to Cervical Cancer, as well as eliminating the HPV-16 and HPV-18 infection that causes these lesions. The REVEAL trials are randomized, double-blind, placebo-controlled trials involving adult women with HPV-16 and HPV-18 positive biopsy-proven cervical HSIL. REVEAL1 provided one-year post-endpoint safety data for at least 198 randomized participants, while REVEAL2 will provide efficacy and one-month safety data for at least 198 participants.
Inovio completed the 52-week safety follow-up of REVEAL1 participants and found that the VGX-3100 safety profile at Week 36 remained well-tolerated through Week 88. Furthermore, participants who received VGX-3100 and met the primary endpoint at Week 36 were free of HPV-16 and/or HPV-18 at Week 88. The per-protocol assessment of primary efficacy did not differ significantly from the mITT analysis previously reported for REVEAL1.
Inovio is also working with QIAGEN to co-develop a liquid biopsy-based diagnostic product based on next-generation sequencing (NGS) technology to help guide clinical decision-making for using VGX-3100 in cervical HSIL. If validated, this biomarker can increase the likelihood of clinical response in biomarker-positive women with cervical HSIL. QIAGEN has extensive expertise in technologies ranging from polymerase chain reaction (PCR) to next-generation sequencing (NGS) for diagnostic development. Inovio expects to have more information on its biomarker development by 2022.
Separately, Inovio’s partner ApolloBio dosed the first patient in the VGX-3100 Phase 3 clinical trial in China. The Phase 3 trial in China is designed similarly to REVEAL1 and REVEAL2 – it is randomized, double-blind, and placebo-controlled. The trial is expected to enroll 84 people. The companies signed an agreement in 2018 that gave ApolloBio the exclusive right to develop, manufacture, and commercialize VGX-3100 in Greater China.
Furthermore, apart from Inovio and ApolloBio, several clinical trials in Cervical Dysplasia are ongoing by key companies such as Brooklyn Immuno Therapeutics (IRX-2), PapiVax [(PVX-2 (pNGVL4a-Sig/E7 [Detox]/HSP70)], Frantz Viral Therapeutics (Artesunate), and others.
Cilta-cel: Janssen: R/R Multiple Myeloma
Drug Name: Cilta-cel
Company: Janssen/Legend Biotech
MOA: Immunologic cytotoxicity; T lymphocyte replacements
Originator: Legend Biotech USA; Nanjing Legend Biotech
Special Status: Breakthrough Therapy Designation for R/R Multiple Myeloma (2019) and Orphan Drug Designation for R/R Multiple Myeloma (2019)
Cilta-cel is a CAR-T cell therapy that targets the BCMA protein, whose overexpression is linked to cancer cell formation. Legend Biotech, Inc. signed an exclusive worldwide license and collaboration agreement with Janssen Biotech, Inc. in December 2017 to develop and commercialize cilta-cel. Legend announced in December 2020 the start of a rolling submission to the FDA of a Biologics License Application seeking approval of cilta-cel for the treatment of Relapsed or Refractory Multiple Myeloma, which was accepted under Priority Review in May 2021. Cilta-cel previously received Breakthrough Therapy Designation in the United States in December 2019, as well as Orphan Drug Designation in February 2019.
The FDA initially set Nov. 2021 as the PDUFA decision date for cilta-cel (ciltacabtagene autoleucel) in Myeloma, but then granted an extension to allow more data to be analyzed from the phase 1/2 CARTITUDE-1 clinical trial, which looked at cilta-cel in adults with Relapsed/Refractory Myeloma.
CARTITUDE-1 results were presented at the American Society of Hematology Annual Meeting in December, revealing that 97.9% of patients had their disease shrink due to the CAR-T cell therapy cilta-cel, with 82.5% experiencing a stringent complete response. A stringent complete response denotes a more in-depth than a complete standard response. The PDUFA decision date has been pushed back to February 28, 2022.
Get insights about the Multiple Myeloma Market Landscape in the next decade
Zolbetuximab: Astellas Pharma: Gastric Cancer
Drug Name: Zolbetuximab
Company: Astellas Pharma
MOA: Antibody-dependent cell cytotoxicity; Immunologic cytotoxicit
Originator: Ganymed Pharmaceuticals
Special Status: Orphan Drug Designation (in EU-2010; in the US- 2012)
Astellas’ experimental oncological drug candidate zolbetuximab is a monoclonal antibody against isoform 2 of claudin 18 (claudin 18.2), a cell adhesion molecule involved in the formation of tight junctions in epithelial cells. When claudin-18.2 is present as a tumor-specific marker, the drug may destroy cancerous tissue via antibody-dependent cellular cytotoxicity.
In November 2010, the European Union granted orphan drug designation to zolbetuximab for the treatment of gastric cancer. In November 2012, this indication was also granted orphan drug status in the United States.
Late-stage research is underway in the Phase III GLOW (NCT03653507) and SPOTLIGHT (NCT03504397) studies, which test the antibody as first-line therapy for HER2-negative disease in combination with the CAPOX and FOLFOX chemotherapy regimens, respectively, in zolbetuximab’s primary indication of Gastric Cancer. Around 20% of gastric adenocarcinomas overexpress HER2, while 70% express claudin-18.2, potentially allowing zolbetuximab to be used by a substantial percentage of patients. Furthermore, treatment options for first-line HER2-negative disease remain limited; the recently approved CheckMate 649 regimen of frontline Opdivo and chemotherapy, while a huge step forward in the field, is primarily limited to PD-L1+ (CPS 5) tumors.
The Phase II FAST (NCT01630083) trial results were promising, with claudin-18.2-positive patients receiving zolbetuximab and chemotherapy showing significant PFS and OS improvements compared to chemotherapy alone. Suppose the upcoming readouts from GLOW and SPOTLIGHT can replicate this benefit. In that case, zolbetuximab may find an extensive and profitable niche in first-line Gastric Cancer treatment, particularly among weakly immunogenic patients, and would respond poorly to PD-1 antibodies like Opdivo. The FDA has granted the agent orphan drug designation primarily for this reason. With Astellas anticipating a regulatory submission in early 2022, zolbetuximab is a strong contender for the first-in-class launch in 2022. Claudin-18.2 may be a target in various solid tumors other than Gastric Adenocarcinomas. Notably, the isoform is expressed in approximately 50% of Pancreatic Cancers, where zolbetuximab is currently in early-stage development. A successful launch in Gastric Cancer treatment would be a step forward in expanding the oncological drug’s indications.
Ilaris: Novartis: NSCLC
Drug Name: Ilaris
Company: Novartis
MOA: Interleukin 1 beta inhibitors
Originator: Novartis
Special Status: Approved for ASOD (2020), SJIA (2013), TRAPS, HIDS, FMF (2016), and CAPS (2009) in the US
Ilaris (canakinumab) developed by Novartis is a human anti-interleukin-1 monoclonal antibody used to treat Periodic Fever Syndromes (Cryopyrin-Associated Periodic Syndromes, Tumor Necrosis Factor Receptor Associated Periodic Syndrome, Hyper immunoglobulin D Syndrome/Mevalonate Kinase Deficiency, Familial Mediterranean Fever) and active Still’s a disease, including Adult-Onset Still’s (SJIA).
Initially approved for autoimmune disorders, Novartis’ IL-1ꞵ monoclonal antibody Ilaris is now being studied as an adjuvant and first-line NSCLC treatment. Novartis launched a comprehensive NSCLC development program for Ilaris after a subgroup analysis from the Phase III CANTOS study in atherosclerosis revealed that it reduced Lung Cancer incidence and mortality.
Despite the recent failure of the Phase III CANOPY-2 trial of Ilaris in the second-line post-checkpoint inhibitor setting, Novartis is hoping that in the Phase III CANOPY-1 trial, Ilaris will demonstrate synergy with first-line standard of care Keytruda and platinum doublet chemotherapy.
MRTX849: Mirati Therapeutics: NSCLC
Drug Name: MRTX849
Company: Mirati Therapeutics
MOA: KRAS protein inhibitors
Originator: Array BioPharma; Mirati Therapeutics
Special Status: None
Mirati Therapeutics is developing Adagrasib (MRTX849), an experimental, highly selective, and potent oral small-molecule treatment to decrease difficult-to-treat tumours with the KRASG12C mutation.
Mirati Therapeutics’ KRAS G12C inhibitor MRTX849 is on track to become the second KRAS inhibitor approved for NSCLC. The Phase I/II KRYSTAL study found that a cohort of pretreated NSCLC patients with a KRAS pg.G12C mutation who received 600mg MRTX849 daily had a 45% overall response rate (ORR) and a 96% disease control rate (DCR). The drug is currently in Phase 2 NSCLC clinical trials. This Phase 2 study will assess the efficacy and safety of MRTX849 as monotherapy and in combination with pembrolizumab. There will be three cohorts of patients who have the KRAS G12C mutation, have advanced or metastatic NSCLC, and are candidates for first-line treatment. Two cohorts with a PDL-1 TPS score of 1% are randomized to MRTX849 monotherapy or MRTX849 combined with pembrolizumab. The third cohort has a PDL-1 TPS score of 1% or higher and is treated with MRTX849 and pembrolizumab.
If approved, this oncological drug would face competition from Amgen’s Lumakras (sotorasib), which has shown similar efficacy results and received FDA approval in May 2021. The competition between these two KRAS inhibitors will most likely come down to the relative risk/benefit profiles of the drugs and which drug enters the market first. Mirati has begun comprehensive development programs for adagrasib-based combinations in both NSCLC and CRC.
Adagrasib (MRTX849) demonstrated promising clinical activity and safety signals in heavily pretreated patients with KRAS G12C–mutated colorectal cancer in the KRYSTAL-1 study (NCT03785249) as a monotherapy or combination with cetuximab (Erbitux) during the ESMO 2021.
Mirati presented new data at ESMO 2021 indicating that their investigational KRAS-candidate Adagrasib could outperform Amgen’s Lumakras, at least in Colon Cancer treatment, even though Lumakras is approved for Lung Cancer but not Colon Cancer.
Find out Who is better? Lumakras or Adagrasib?
Apart from Amgen and Mirati, several other companies such as Cardiff Oncology, Verastem, Onconova Therapeutics, Bristol-Myers Squibb, Boehringer Ingelheim, Revolution Medicines, Novartis Pharmaceuticals, InventisBio Inc., Merck Sharp & Dohme Corp, Moderna, and others are also evaluating their respective KRAS inhibitors in clinical trials.
Jemperli: GSK: Ovarian Cancer
Drug Name: Jemperli
Company: GSK
MOA: Antibody-dependent cell cytotoxicity; Programmed cell death-1 receptor antagonists; T lymphocyte stimulants
Originator: AnaptysBio
Special Status: None
Jemperli developed by GSK, is a programmed death receptor-1 (PD-1)–blocking antibody that works by binding to PD-1 and inhibiting PD-1 interactions with PD-L1 or PD-L2. Jemperli has been approved in Canada as a monotherapy for treating adult patients with Mismatch Repair Deficient (dMMR) or Microsatellite Instability-High (MSI-H) recurrent or advanced Endometrial Cancer that has progressed on or after prior treatment with a platinum-containing regimen.
Jemperli’s conditional approval is based on the findings of the multi-cohort GARNET study. Jemperli is on track to become the first PD-1/PDL1 inhibitor approved for Ovarian Cancer patients. Apart from the GARNET study, Jemperli and Zejula are also being tested in the Phase III FIRST study to treat advanced Ovarian Cancer in both first-line and first-line maintenance settings. A future label expansion into the more lucrative first-line setting would increase Jemperli’s overall commercial potential. However, it will almost certainly have to compete with the other checkpoint inhibitors in development for this treatment setting.
Lutetium 177LU-PSMA-617: Novartis: Prostrate Cancer
Drug Name: Lutetium 177LU-PSMA-617
Company: Novartis
MOA: Ionising radiation emitters
Originator: Endocyte; Radboud University; RadioMedix
Special Status: Priority Review for mCRPC (2021)
177Lu-PSMA-617 is a PSMA-targeted radioligand therapy under investigation for metastatic Castration-Resistant Prostate Cancer. It is a type of precision cancer treatment in which a targeting compound (ligand) is combined with a therapeutic radioisotope (a radioactive particle). After being administered into the bloodstream, 177Lu-PSMA-617 attaches to prostate cancer cells that express PSMA14, a transmembrane protein, and has a high tumor-to-normal tissue absorption. Once bound, the radioisotope’s emissions harm tumor cells, interfering with their ability to replicate and causing cell death. The radioisotope’s radiation travels over very short distances, causing minimal damage to surrounding cells.
The FDA granted priority review to an NDA in 2021 for the investigational targeted radioligand therapy 177Lu-PSMA-617 to treat patients with mCRPC who have previously received androgen-receptor pathway and taxane-based chemotherapy. The Prescription Drug User Fee Act deadline is set for the first half of 2022.
Priority review was granted on the basis of positive data from the phase 3 VISION study (NCT03511664), which compared 177Lu-PSMA-617 plus protocol-permitted standard-of-care (SOC) therapy to SOC alone in patients with progressive prostate-specific membrane antigen (PSMA)–positive mCRPC. The FDA previously granted 177Lu-PSMA-617 Breakthrough Therapy Designation in June 2021 for mCRPC treatment based on data from the same phase 3 VISION study.
Furthermore, PSMAfore (NCT04689828) and PSMAddition (NCT04689829) are two ongoing phase 3 studies examining 177Lu-PSMA-617 in earlier lines of therapy for patients with mCRPC. PSMAfore is comparing 177Lu-PSMA-617 to androgen receptor–directed therapy in patients with advanced mCRPC who have not received taxane-based chemotherapy. PSMAddition compares 177Lu-PSMA-617 plus SOC to SOC alone in metastatic hormone-sensitive Prostate Cancer.
Explore our newsletter to get more details about the Prostate Cancer Pipeline Landscape
Imbruvica + Venclexta: AbbVie: CLL/MCL
Drug Name: Imbruvica
Company: AbbVie
MOA: Agammaglobulinaemia tyrosine kinase inhibitors; Emt protein-tyrosine kinase inhibitors
Originator: Celera Genomics Group
Special Status: Approved for MCL (2013), CLL (2014), Waldenstrom’s Macrogobulinemia (2015), cGvHD (2017), and MZL (2017) in the US
Drug Name: Venclexta
Company: AbbVie
MOA: Apoptosis stimulants; Proto-oncogene protein c-bcl-2 inhibitors
Originator: Abbott Laboratories; Genentech; Walter and Eliza Hall Institute of Medical Research
Special Status: Approved for CLL (2016) in the US
Imbruvica, a Bruton’s tyrosine kinase (BTK) inhibitor, and Venclexta, an inhibitor of the anti-apoptotic protein BCL-2, are two AbbVie’s blockbuster oncological drugs being developed as new CLL and MCL combinations. Imbruvica is approved in over 100 countries and has been used to treat over 250,000 patients globally. More than 50 company-sponsored clinical trials, including 18 Phase 3 trials, have been done to evaluate Imbruvica’s efficacy and safety during the past 11 years. The FDA first approved Imbruvica in November 2013, and it is now approved for adult patients in six disease areas, including five hematologic cancers. In contrast, the FDA approved Ventoclax in 2016 for CLL treatment.
Imbruvica monotherapy is favored as first-line therapy in CLL, with Venclexta with anti-CD20 rituximab as the optimal strategy for patients who have relapsed following a BTK inhibitor. Long-term follow-up data from the RESONATE-2 trial showed Imbruvica had a 70% PFS at five years, establishing it as a transformative first-line therapy. While Imbruvica is highly effective at disease control, the best responses are typically partial remissions, and patients must continue treatment to maintain disease control.
Furthermore, two trials were done to examine the efficacy and safety of Imbruvica(ibrutinib) plus venetoclax (I+V) as a prospective fixed-duration treatment in adult patients with previously untreated Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma, . Both of these triasls were presented at the ASH 2021. The new secondary endpoint data from the Phase 3 GLOW study (NCT03462719) revealed that fixed-duration I+V treatment resulted in deeper undetectable minimal residual disease (uMRD) responses compared to patients treated with chlorambucil plus obinutuzumab (Clb+O), and additional analysis revealed that uMRD responses were better sustained during the first year post-treatment.
Updated results from the same investigational regimen’s Phase 2 CAPTIVATE study (NCT02910583), now with a median 38-month follow-up, demonstrated sustained uMRD and disease-free survival (DFS). There were no new MRD relapses, clinical progressions, or deaths after an additional year of study follow-up in patients with confirmed uMRD who were randomized to placebo or continued Imbruvica after 12 cycles of combined I+V.
Based on ORR data, Imbruvica received accelerated approval as a second-line or later treatment for MCL in November 2013. A Phase III trial, RAY, confirmed Imbruvica’s efficacy by demonstrating superior PFS and ORR rates when compared to Toricel, which is approved for MCL in the EU but not in the US. Venclexta has shown single-agent activity in MCL, but it is not yet approved for this indication.
In second-line or later MCL, SYMPATICO compares Imbruvica + Venclexta to Imbruvica alone. A regulatory submission in early 2022 is expected if the results are positive, followed by a possible approval in late 2022. If this combination is approved, it could become the new standard of care for patients who have failed first-line chemotherapy.
TG-1303: TG Therapeutics: CLL
Drug Name: Ukoniq
Company: TG Therapeutics
MOA: Casein kinase Iepsilon inhibitors; Phosphatidylinositol 3 kinase delta inhibitor
Originator: Incozen Therapeutics; Rhizen Pharmaceuticals
Special Status: Approved for MZL and FL (2021) in the US; BLA in combination with Ublituximab for CLL (2021) in the US
Drug Name: Ublituximab
Company: TG Therapeutics
MOA: Antibody-dependent cell cytotoxicity; T lymphocyte stimulants
Originator: LFB Biotechnologies; rEVO Biologics
Special Status: BLA in combination with Ukoniq for CLL (2021) in the US
TG-Therapeutics TG-1303 is a combination of Ukoniq, a PI3K delta inhibitor, and ublituximab, an anti-CD20 antibody. Ukoniq is administered once daily, whereas other oral, approved PI3K inhibitors are administered twice daily. Ukoniq also inhibits CK1 epsilon, a potential Treg count and function regulator, which may improve its safety profile. Ukoniq recently got the FDA approval for MZL and FL treatment in 2021. On the other hand, Ublituximab has been glycoengineered for increased potency and is distinguished by its shorter infusion times when compared to other approved antibodies in this class. TG Therapeutics has completed a BLA submission for TG-1303 for first-line and R/R CLL in 2021, and an approval decision is expected in March 2022.
The UNITY-CLL trial, which enrolled first-line and second-line or later CLL patients and compared TG-1303 to Gazyva combined with chlorambucil, supported the BLA submission. While the Ukoniq combination had a longer PFS (32 months vs. 18 months), it is no longer widely used in CLL. As a result, the 38-month median PFS for first-line patients can be compared to previous data for Imbruvica monotherapy, which reported a 70% PFS for first-line patients at 60 months (median PFS was not reached). In the MURANO trial, Venclexta combined with rituximab had a PFS of 53.6 months for relapsed/refractory patients, whereas Ukoniq alone had a PFS of 19.5 months. If approved, the Ukoniq combination will most likely be used in the third-line or later setting, competing with Gilead’s Zydelig and Secura Bio’s Copiktra based on safety.
Kymriah, Breynzi and Yescarta: Novartis, BMS and Gilead Sciences: DLBCL
Drug Name: Kymriah
Company: Novartis
MOA: Immunologic cytotoxicity; T lymphocyte replacements
Originator: Lentigen Corporation; University of Pennsylvania
Special Status: Approved for third-line or later DLBCL (2018)
Drug Name: Yescarta
Company: Gilead Sciences
MOA: Immunologic cytotoxicity; T lymphocyte replacements
Originator: Cabaret Biotech
Special Status: Approved for third-line or later DLBCL (2013)
Drug Name: Breynzi
Company: Bristol-Myers Squibb
MOA: Immunologic cytotoxicity; T lymphocyte replacements
Originator: Juno Therapeutics
Special Status: Approved for third-line or later DLBCL (2021)
Kymriah developed by Novartis is a chimeric antigen receptor T-cell (CAR-T) therapy that reprogrammes the patient’s T-cells with a transgene encoding a CAR while identifying and removing CD19-expressing malicious cells. While Gilead’s Yescarta (axicabtagene ciloleucel) is a genetically modified autologous T cell immunotherapy that targets CD19.
The three oncological drugs, Yescarta, Kymriah, and Breyanzi, were approved for third-line or later DLBCL in October 2017, May 2018, and February 2021, respectively, based on single-arm trials. While all of these CD19-directed autologous CAR-T therapies are thought to be potentially curative, Yescarta stands out due to its higher efficacy and shorter manufacturing time and thus is used in more patients. Kymriah is distinguished by its improved safety and is prescribed to elderly patients. Breyanzi, which has the best safety profile of the three and efficacy comparable to Yescarta, is only now beginning to enter the DLBCL market.
BELINDA, ZUMA-7, and TRANSFORM are Phase III trials evaluating Kymriah, Yescarta, and Breyanzi in second-line DLBCL patients eligible for transplantation, respectively. Patients in the treatment arm of these trials receive lymphodepleting chemotherapy plus CAR-T therapy. In contrast, those in the comparator arm receive several cycles of platinum-based immunochemotherapy followed, in responding patients, by high-dose chemotherapy plus autologous stem cell transplant. Patients in BELINDA can receive platinum-based chemoimmunotherapy prior to lymphodepleting chemotherapy + Kymriah.
The Novartis’ BELINDA trial did not meet its primary endpoint of event-free survival when compared to standard-of-care treatment (SOC). SOC was salvage chemotherapy, followed by high-dose chemotherapy and stem cell transplant in responding patients. The safety profile was consistent with Kymriah’s established safety profile. Novartis will conduct a thorough analysis of the BELINDA data and work with investigators to deliver future findings.
Moreover, according to top-line results from the primary analysis of Gilead’s ZUMA-7 study, Yescarta (axicabtagene ciloleucel) outperformed standard of care (SOC) in second-line R/R Large B-cell Lymphoma. The primary endpoint of event-free survival of the trial after a median follow-up of two years was achieved. The study also achieved the crucial secondary endpoint of objective response rate (ORR). The interim study of overall survival (OS) demonstrated a tendency in favour of Yescarta; however, the data is still in early stages, and more analyses are planned in the future.
Furthermore, BMS’ TRANSFORM study is scheduled to be completed in January 2024. Moving CAR-T therapy to earlier lines may result in healthier CAR-T cells, better response rates, and avoiding the toxicity of standard chemotherapy. On the other hand, stem cell transplant has the advantage of physician familiarity and has the potential to be curative. Before CAR-T therapy can be used to replace transplants, doctors will most likely want to see long-term survival data. A supplementary approval for second-line DLBCL would also broaden the pool of patients eligible for CAR-T therapy, and it is expected for Kymriah and Yescarta in late 2022.

Parsaclisib: Incyte: FL, MCL, and MZL
Drug Name: Parsaclisib
Company: Incyte
MOA: Phosphatidylinositol 3 kinase delta inhibitors
Originator: Incyte Corporation
Special Status: Priority Review for MCL and MZL (2021)
Parsaclisib developed by Incyte is a new oral phosphatidylinositol 3-kinase delta inhibitor intended to avoid the hepatotoxicity associated with first-generation inhibitors. CITADEL203, 204, and 205 are pivotal Phase II trials evaluating parsaclisib for third-line or later FL and second-line or later MZL or MCL (with or without a prior BTK inhibitor). To decrease toxicity in the CITADEL studies, parsaclisib was given at a dose of 20 mg once daily for eight weeks, then either 20 mg once weekly or 2.5 mg once daily. The 2.5 mg step-down dose produced better interim results, with an ORR of 75% and a CR of 13.5 % for FL, an ORR of 66%, a CR of 11%, and a PFS of 11 months for MCL, and an ORR of 58% and a PFS of 11.5 months for MZL. Incyte filed an NDA for Relapsed/Refractory NHL based on the revised CITADEL trial findings in November 2021. Furthermore, a Phase III CITADEL-302 trial will evaluate parsaclisib in combination with an anti-CD20 for R/R FL and MZL, while CITADEL-310 will evaluate parsaclisib in combination with bendamustine and rituximab for newly diagnosed MZL.
Moreover, the FDA granted parsaclisib priority review for two lymphoma indications in 2021. The designations apply to adults with relapsed or refractory Marginal Zone Lymphoma who have previously received at least one anti-CD-20-based regimen and adults with mantle cell lymphoma who have previously received at least one anti-CD-20-based regimen. In addition, the FDA will conduct a routine review of the agent for treating individuals with R/R FL who have had at least two prior systemic therapies. According to the lymphoma studies, parsaclisib had a manageable safety profile and appeared to be generally well-tolerated. By April 30, 2022, the FDA is expected to make a decision on the Marginal Zone and Mantle Cell Lymphoma indications. In contrast, the FDA is expected to make a decision on the Follicular Lymphoma indication by August 30, 2022.
Furthermore, several companies such as Eisai, Bayer, Gilead Sciences, BMS, Roche, TG Therapeutics, Verastem Oncology, and others are also performing clinical trials in Follicular Lymphoma and evaluating their drug candidates to give tough competition to Incyte.
Navitoclax: AbbVie/Roche: Myelofibrosis
Drug Name: Navitoclax
Company: AbbVie/Roche
MOA: Apoptosis stimulants; Proto-oncogene protein c-bcl-2 inhibitors
Originator: Abbott Laboratories
Special Status: None
Navitoclax is a BCL-XL/BCL-2 inhibitor being studied for myelofibrosis treatment. The oncological drug navitoclax, developed by AbbVie and Roche, is a Venclexta analog. Venclexta inhibits only BCL-2, while navitoclax inhibits a broader range of anti-apoptotic proteins, including BCL-2, BCL-XL, and BCL-W. A pivotal Phase II trial is evaluating navitoclax in combination with Jakafi in MF patients who have previously taken Jakafi. Interim trial results showed that 29% of patients had a spleen volume reduction (SV35) of 35% from baseline, while six patients (25%) had bone marrow fibrosis improvement. In MF, the abnormal cell population secretes various cytokines and growth factors, which cause marrow fibrosis and stromal changes. The decrease in bone marrow fibrosis could be interpreted as evidence that navitoclax has disease-modifying properties, which Jakafi did not have. AbbVie anticipates a regulatory submission in 2022 and possible accelerated approval in late 2022. Two Phase III trials are currently underway, with results expected in early 2022. TRANSFORM-2 will compare navitoclax + Jakafi to best available therapy in patients with relapsed/refractory MF, while TRANSFORM-1 will compare navitoclax + Jakafi to Jakafi in patients who have never taken Jakafi.
Lynparza: AstraZeneca/Merck: Prostrate Cancer
Drug Name: Lynparza
Company: AstraZeneca/Merck
MOA: Poly(ADP-ribose) polymerase inhibitors
Originator: KuDOS Pharmaceuticals; University of Pennsylvania
Special Status: Approved for Ovarian, Breast, Pancreatic, and Prostate Cancer
Lynparza (olaparib) developed by AstraZeneca is a first-in-class PARP inhibitor and the first targeted treatment to block DNA damage response (DDR) in cells/tumors with a defect in homologous recombination repair (HRR), such as those with BRCA1 and BRCA2 mutations, or those where deficiency is induced by other agents (such as NHAs).
AstraZeneca is evaluating Lynparza in the first-line setting of mCRPC patients in the Phase III PROpel study alongside abiraterone versus placebo plus abiraterone. The PROpel study found that combining Lynparza (olaparib) and Johnson & Johnson’s hormonal therapy Zytiga (abiraterone acetate) as a first-line mCRPC delayed disease progression treatment for men. Lynparza was approved last year as second-line therapy for Homologous Recombination Repair (HRR) gene-mutated mCRPC, but expanding its label to include non-HRR patients in the frontline setting would significantly expand the number of patients eligible for treatment. Both the companies stated that they will share the new information with regulatory authorities soon.
This would provide the companies an advantage over competing PARP medications like Clovis Oncology’s Rubraca (rucaparib), which was approved for Prostate Cancer with a restricted label last year but won’t publish more clinical data until 2022, as well as GlaxoSmithKline’s Zejula (niraparib) and Pfizer’s Talzenna (talazoparib). Lynparza is already a big earner for AstraZeneca and Merck thanks to its earlier approvals in Ovarian, Breast, Pancreatic, and Prostate Cancer.
Tebentafusp: Immunocore: Uveal Melanoma
Drug Name: Tebentafusp
Company: Immunocore
MOA: Immunologic cytotoxicity; T lymphocyte stimulants
Originator: Immunocore
Special Status: BLA and Marketing Authorization Application for metastatic Uveal Melanoma; Orphan Drug Designation for mUM (2016)
Tebentafusp is a bispecific t-cell engager (BiTE) whose development represents a significant breakthrough in cancer therapy. The agent binds to lymphocyte CD3 receptors, and gp100 antigens expressed on cancer cells, physically bringing T-cells to tumor cells in a mechanism unique to solid tumor indications.
The FDA has designated tebentafusp as an orphan drug in January 2016 for the treatment of Uveal Melanoma. Furthermore, the FDA and the European Medicines Agency have approved a BLA and a marketing authorization application for tebentafusp (IMCgp100) for HLA-A 02:01–positive metastatic Uveal Melanoma treatment. The submissions are based on the findings of the phase 3 IMCgp100-202 trial (NCT03070392), which evaluated tebentafusp in patients with previously untreated metastatic Uveal Melanoma. The FDA previously designated tebentafusp as a breakthrough therapy for adult patients with HLA-A 02:01–positive unresectable or metastatic Uveal Melanoma.
The trial’s findings were presented at the AACR Annual Meeting 2021, and it showed that after a median follow-up of 14.1 months, patients treated with the experimental agent had a median OS of 21.7 months (95% CI, 18.6-23.6), compared to the investigator’s choice of pembrolizumab (Keytruda), ipilimumab (Yervoy), or dacarbazine, which had a median OS of 16.0 months (95% In addition, the tebentafusp arm had a 73.2% one-year OS rate against 58.5% in the control arm.
The priority review for the novel T-cell receptor bispecific immunotherapy drug might cut the review time in half, from ten to six months, from the date of filing acceptance. As a result, the anticipated Prescription Drug User Fee Act implementation date is February 23, 2022.
Mosunetuzumab: Roche: Follicular Lymphoma
Drug Name: Mosunetuzumab
Company: Roche
MOA: Antibody-dependent cell cytotoxicity; Cytotoxic T lymphocyte stimulants
Originator: Genentech
Special Status: Breakthrough Therapy Designation for FL (2020)
Mosunetuzumab is a CD20xCD3 T-cell engaging bispecific that targets CD20 on the surface of B-cells and CD3 on the surface of T-cells. By releasing cytotoxic proteins into the B-cells, this dual targeting activates and redirects a patient’s existing T-cells to engage and eliminate target B-cells.
The FDA granted Roche’s mosunetuzumab Breakthrough Therapy Designation for third-line or later FL in July 2020. GO29781, a Phase I/II trial, is enrolling several cohorts, including one of third-line or later FL patients. Mosunetuzumab, if approved, has the potential to be a first-in-class CD20xCD3 T-cell engaging bispecific antibody in NHL.
Mosunetuzumab was presented for the first time at the 63rd ASH Annual Meeting and Exposition held in December. Emerging data show that mosunetuzumab has a promising benefit-risk profile in R/R Follicular Lymphoma treatment, a slow-growing, or indolent, form of NHL. The pivotal findings of the phase I/II GO29781 trial showed that mosunetuzumab induces durable CR lasting at least 18 months in heavily pretreated individuals with R/R Folicular Lymphoma who have received 2 or more prior therapies, with a 60.0% CR rate and a median progression-free survival of 17.9 months in heavily pretreated patients with R/R FL who have received two or more prior therapies (95 percent CI: 10.1-not evaluable). Respondents’ median response time was 22.8 months (95 % CI: 9.7-not evaluable). The most common AE was cytokine release syndrome (CRS), which was generally of low severity (mainly Grade 1-2).
Roche recently submitted an initial marketing authorization application for mosunetuzumab to the European Medicines Agency, intending to bring this drug to people with NHL as soon as possible. Genentech planing to submit the new data to FDA for approval consideration in the near future.
Sabatolimab: Novartis: MDS
Drug Name: Sabatolimab
Company: Novartis
MOA: Antibody-dependent cell cytotoxicity; HAVCR2 protein inhibitors; T lymphocyte stimulants
Originator: Novartis
Special Status: Fast Track Designation for MDS in the US (2021); Orphan Drug Designation for MDS in EU (2021)
Sabatolimab (MBG453) from Novartis is a potentially first-in-class antibody that targets TIM-3. TIM-3 is found on T-cells, where it inhibits T-cell activation, and on leukemic stem cells (LSC). Hypomethylating agents have been shown to increase the expression of TIM-3 on LSC. Hypomethylating agents, such as azacitidine and decitabine, have been used to treat MDS for over a decade and have resulted in a modest survival benefit.
The FDA granted sabatolimab (MBG453) fast track designation in May 2021 to treat adult patients with Myelodysplastic Syndromes (MDS) with an IPSS-R risk category of high or very high risk in combination with hypomethylating agents. Whereas the European Commission granted Sabatolimab the Orphan Drug Designation in August 2021.
According to results presented at the 63rd ASH Annual Meeting and Exposition 2021, Sabatolimab (MBG453) induced a median DOR greater than one year in patients with very high/high-risk MDS and AML when used in combination with hypomethylating agents. The overall response rate (ORR) in the MDS arm (n = 51) was 56.9 %, with 19.6 % complete responses (CR). Brunner went on to say that 23.5 % of the patients had marrow CR (mCR), half of which had mCR with hematologic improvement (mCR-HI). The average DOR was 17.1 months (95 % CI, 6.7-not evaluable [NE]). The median DOR for patients with a CR was 19.3 months (95 % CI, 12.1-NE) and 7.9 months (95 % CI, 3.0-NE) for those with mCR-HI.
Currently, multiple studies in the STIMULUS clinical trial program are examining sabatolimab as part of different combination therapy in patients with Myelodysplastic Syndromes and Acute Myeloid Leukemia, including the Phase II STIMULUS-MDS1, Phase III STIMULUS-MDS2, Phase II STIMULUS-MDS3, and Phase II STIMULUS-AML1 studies. Phase III STIMULUS-MDS2 is similar to STIMULUS-MDS1 in that it enrolls more patients (500 versus 127), and has overall survival as the primary endpoint. It has a primary completion date of May 2027. There are no class competitors being developed for sabatolimab in MDS.
Copiktra: Secura Bio: PTCL
Drug Name: Copiktra
Company: Secura Bio
MOA: Phosphatidylinositol 3 kinase delta inhibitors; Phosphatidylinositol 3 kinase gamma inhibitors
Originator: Intellikine
Special Status: Approved for R/R CLL/SLL and FL (2018); Fast Track Designation for PTCL (2021)
Copiktra developed by Secura Bio is an oral phosphoinositide 3-kinase (PI3K) inhibitor and the first FDA-approved dual inhibitor of PI3K-delta and PI3K-gamma, two enzymes known to promote malignant cell growth and survival. PI3K signaling is thought to play a role in forming and maintaining a supportive tumor microenvironment and may lead to the proliferation of malignant cells. Copiktra is approved for for Relapsed or Refractory Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma treatment in adult patients and for Follicular Lymphoma treatment after at least 2 prior therapies in the United States in 2018. Copiktra is also being developed for Peripheral T-cell Lymphoma (PTCL) treatment, for which it has been granted Fast Track status in the US in 2021.
At the 63rd ASH Annual Meeting, new data for the treatment of Relapsed or Refractory PTCL patients with Copiktra were presented. As per interim analysis of the phase 2 PRIMO trial, the patients with R/R PTCL treated with single-agent Copiktra 75mg BID for the first two months, followed by 25mg BID until progression or unacceptable toxicity. With a minimum follow-up of 6 months, 78 of a planned 125 patients were included in this analysis. The interim results also showed an ORR of 50% (39/78 pts) and a CR of 32.1 % (25/78), with a median DOR of 233 days (range, 1-420+). Patients had a median of three prior therapeutic regimens (range, one to seven) and were diagnosed with the following PTCL subtypes: PTCL NOS (53.8%), ALCL (14.1%), AITL (26.0%), and Other (0.5% ). 18% of patients continue on therapy, 47.4% discontinue due to Parkinson’s disease, 6.4% discontinue for stem cell transplant, and 19.2% terminate due to unacceptable toxicity. The trial is still ongoing, with a completion date of 2022 expected.
What’s Ahead?
Every year regulatory authorities approve many oncological drugs for various indications. Even last year, the FDA has approved 50 drugs, out of which 16 were oncological drugs. The oncological drug pipeline is looking very promising this year too. A number of oncological drugs are under clinical trials and are soon to be launched in the market. All these drugs are of different classes and MoAs. Several oncological drugs such as MRTX849, Lynparza, Mosunetuzumab, Navitoclax, Kymriah, Yescarta, and others have the potential to become blockbuster oncological drugs and give tough competition to others. With the robust oncological drug pipeline, we can witness remarkable growth in the oncological sector this year.
FAQs
Leading companies such as Amgen, Mirati, Cardiff Oncology, Verastem, Onconova Therapeutics, Bristol-Myers Squibb, Boehringer Ingelheim, Revolution Medicines, Novartis Pharmaceuticals, InventisBio Inc., Merck Sharp & Dohme Corp, Moderna, and others are evaluating their respective KRAS inhibitors in clinical trials.
The leading players such as Brooklyn Immuno Therapeutics, PapiVax, Frantz Viral Therapeutics, ApolloBio, Inovio, QIAGEN, and others are evaluating their drug candidates for Cervical Dysplasia treatment.
Notable pharma companies such as Incyte, Eisai, Bayer, Gilead Sciences, BMS, Roche, TG Therapeutics, Verastem Oncology, and others are currently working on their lead assets to improve the Follicular Lymphoma treatment outlook.
Downloads
Article in PDF
Recent Articles
- AstraZeneca’s Tropion-Lung01 Phase III Trial Positive Outcomes; Biogen’s LEQEMBI South Korea Appr...
- CereVasc’s eShunt System Study; FDA Approves NGS-Based CDx for Trastuzumab Deruxtecan; Nanopath S...
- ASH 2022 Annual Meeting – Insights Into Major Diffuse Large B-cell Lymphoma (DLBCL) Abstrac...
- Major Highlights of Follicular Lymphoma (FL) in ASH 2021
- Top 10 Expected Oncology Drug Launches in 2023