AAV Vectors in Gene Therapy: How Recent Clinical Advances are Unraveling New Potentials?
Aug 19, 2022
Table of Contents
There has been a renaissance in gene therapy attempts, spurred partly by the discovery and understanding of novel gene delivery vectors. Adeno-associated virus (AAV) is a non-enveloped virus that may be designed to carry DNA to target cells and has sparked considerable interest in the area, particularly in clinical-stage experimental therapy techniques. The capacity to create recombinant AAV particles devoid of viral genes but carrying DNA sequences of interest for diverse therapeutic purposes has thus far proven to be one of the safest gene therapy techniques.
Adeno-associated virus (AAV), discovered 5 decades back in mid-1960s from laboratory adenovirus (AdV) preparations was a result of serendipity. A few research organizations set out to grasp fundamental AAV biology out of sheer scientific interest and without recognizing its enormous potential as a human gene therapy platform. Several essential characteristics of AAV were described over the first 15-20 years of AAV study, including its genomic configuration and composition, DNA replication and transcription, virulence, and virion assembly.
Downloads
Article in PDF
Recent Articles
- Plasma Proteins Therapeutics: Transforming Treatment in Hematology and Beyond
- Vedanta raises; Relay Therapeutics reels in; Gene therapy for SMA
- Biogen’s Aduhelm; FDA Approves Sanofi’s Enjaymo; NHS & Orchard Signs a Deal; Bri...
- AbbVie Receives Approval; Kymriah Approved; Shire Gets USFDA Approval; NICE Rejects; Bristol-Myer...
- Novo Nordisk’s Concizumab for Hemophilia; AbbVie Ends its Alliance with Alector; ADC Therapeutics...
Adeno-Associated Virus (AAV) Vector Designs
The package size of the expression cassette that will be inserted between the two inverted terminal repeats (ITRs) is the most important factor to consider in the logical rAAV vector design. As a starting point, it is widely considered that anything less than 5 kb (including viral ITRs) is enough. Attempts to generate rAAV vectors with packaging cassettes larger than 5 kb result in a significant drop in viral production yields or transgene recombination (truncations). As a result, big coding sequences, such as full-length dystrophin genes, will not be packed well in AAV vectors.
Adeno-associated virus type-2 (AAV2) was one of the first AAV serotypes to be found and described, including its genomic sequence. Because of this early work’s deep understanding of AAV2 biology, most rAAV vectors developed today use the AAV2 ITRs in their AAV vector designs. A mammalian promoter, gene of interest, and a terminator are normally inserted between the ITRs.
In many circumstances, robust, constitutively active promoters are desired for high-level gene expression. The cytomegalovirus (CMV) promoter/enhancer, elongation factor 1a (EF1a), simian virus 40 (SV40), chicken -actin, and CAG (CMV, chicken -actin, rabbit -globin) are all examples of this type of promoter. In most cell types, all of these promoters enable constitutively active, high-level gene expression. Because some of these promoters are susceptible to silencing in specific cell types, this factor must be considered for each application.
AAV as a Gene Therapy Vector
Any viral vector must take various factors into account, including the capacity to adhere to and penetrate the target cell, effective transfer to the nucleus, sustained expression in the nucleus, and a general lack of toxicity. All of these conditions have been met with great success by AAV vectors. Furthermore, a number of improvements have contributed to improve their usability.
Several factors have influenced the creation of modern AAV vector gene therapy, most notably the wild-type virus’s lack of pathogenicity and persistence. Because of the limited size of the AAV genome and concerns about Rep’s possible impact on cellular gene expression, AAV vectors that do not encode Rep and lack the cis-active IEE required for frequent site-specific integration were developed. The ITRs are retained since they are necessary for packing. As a result, modern recombinant AAV (rAAV) vector gene therapy are mostly found as extrachromosomal elements.
Promising AAV Vectors for Gene Therapy in Pipeline
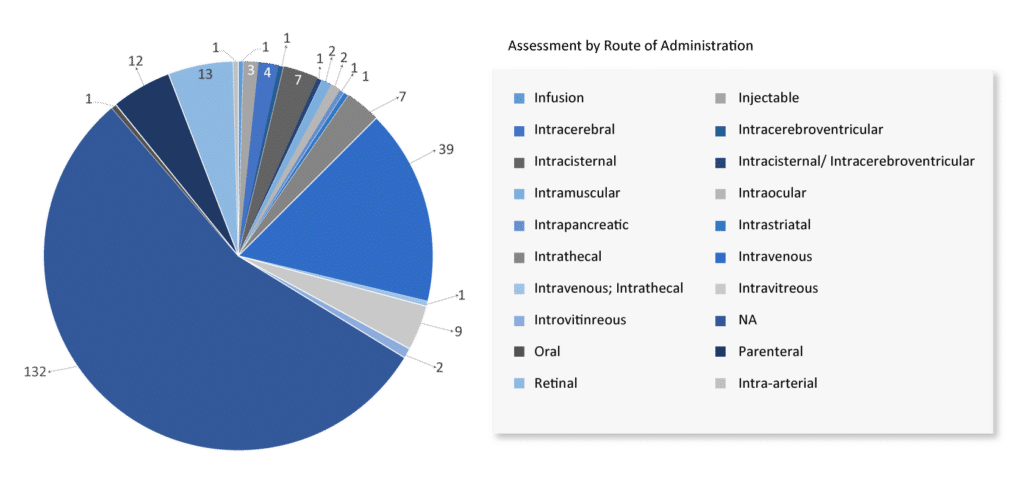
AAV has been found to be a very stable vector, able to survive extensive temperature and pH variations with little to no activity loss. To date, the sole constraint appears to be the concentration with which it is currently being formulated and optimized at about 5 x 1013 particles per milliliter. With the return of clinical usage, this formulation restriction will most certainly be surpassed in the near future. However, the strong stability of these vectors affords enough possibilities to test various administration routes and specific delivery techniques.
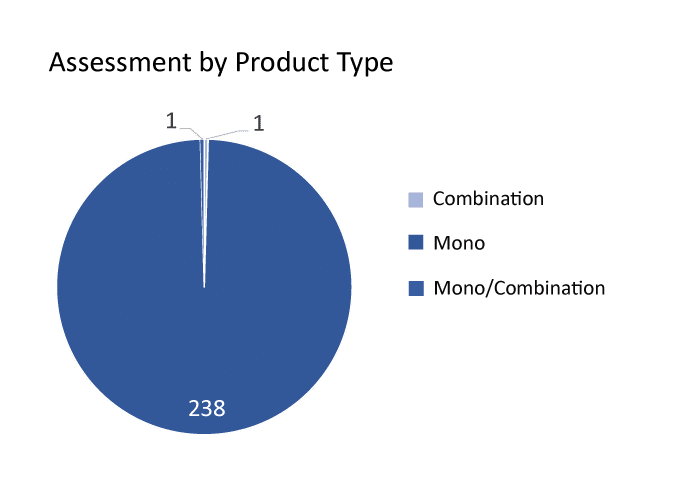
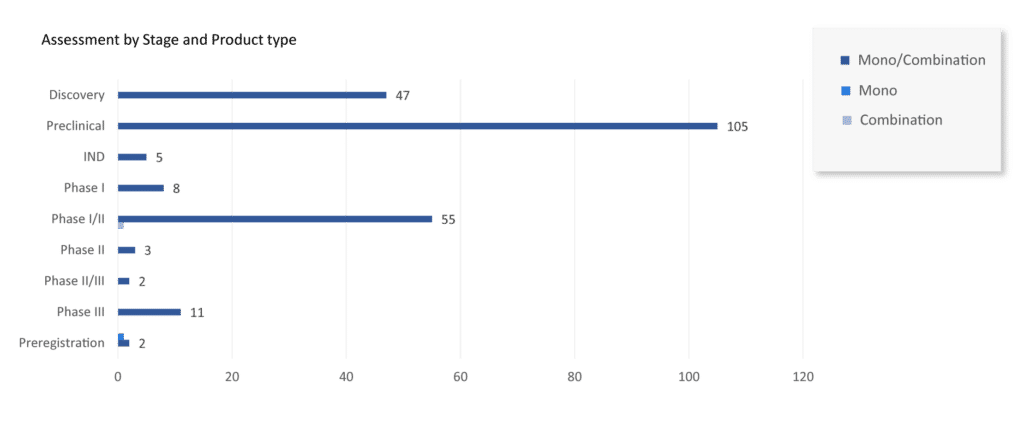
Currently, more than 70 companies are evaluating 235+ AAV vectors in gene therapy landscape. These AAV vectors in gene therapy are presently being studied at different stages of development by leading goliaths such as BioMarin Pharmaceutical, Gensight Biologics, PTC Therapeutics, Ultragenyx Pharmaceutical, MeiraGTx, Pfizer, Biogen, UniQure, Pfizer, Ultragenyx Pharmaceutical, REGENXBIO, Biogen, Spark Therapeutics (Roche), Sarepta Therapeutics, Neurophth Therapeutics, LYSOGENE, Gyroscope Therapeutics, Nanoscope Therapeutics, Homology Medicines, Ultragenyx Pharmaceutical, Passage Bio, Freeline Therapeutics, Astellas Pharma, Aspa Therapeutics, Adrenas Therapeutics, ESTEVE, Sio Gene Therapies, Amicus Therapeutics, 4D Molecular Therapeutics, and others.
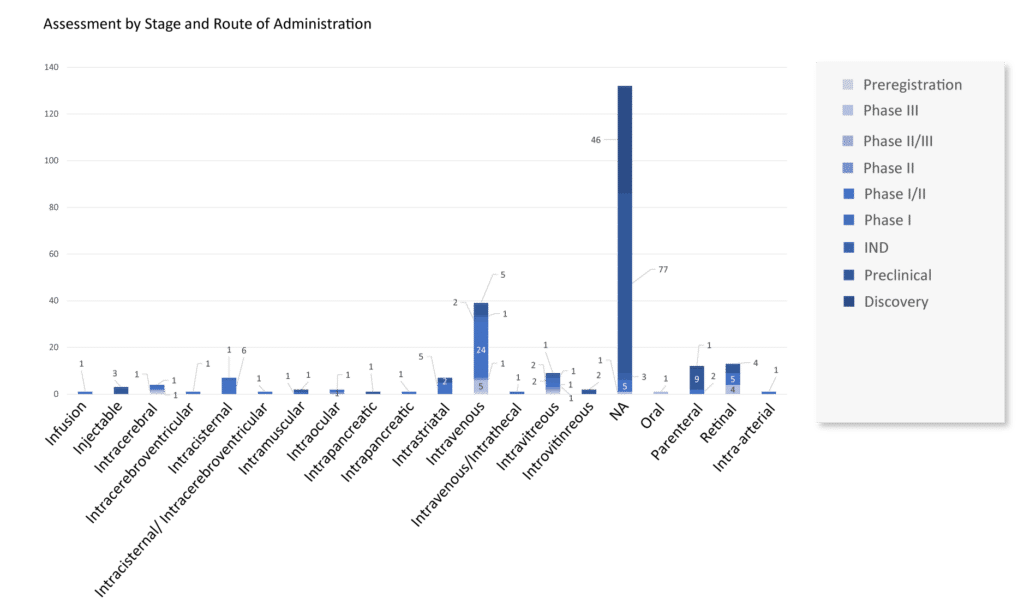
Every AAV vector gene therapy candidate has a novel MoA and product type. The most promising AAV vector for gene therapy in the pipeline includes:
GS010: Gensight Biologics
LUMEVOQ® (GS010; Lenadogene Nolparvovec) targets Leber Hereditary Optic Neuropathy (LHON) by leveraging a mitochondrial targeting sequence (MTS) proprietary technology platform developed at the Institut de la Vision in Paris, which, when associated with the gene of interest, allows the platform to specifically address mitochondrial defects using an AAV vector. The gene of interest is introduced into the cell and expressed, resulting in the production of a functional protein which is subsequently shuttled to the mitochondria via particular nucleotidic sequences to restore the missing or defective mitochondrial function. The European Medicines Agency (EMA) approved “LUMEVOQ” as the invented name for GS010 (lenadogene nolparvovec) in October 2018.
The European Marketing Authorisation Application for LUMEVOQ® is currently being reviewed, with the CHMP’s decision expected in Q3 2023 as a consequence of an extension granted by the EMA for GenSight’s replies to its Day 120 queries. Commercialization will begin after approval by the end of 2023.
Product Development Activities
Presentation of GS010 Clinical Data at EUNOS 2022
GenSight Biologics stated in June 2022 that it will attend the EUNOS 2022 meeting, which will be held on June 20-23 in Birmingham, United Kingdom.
European Regulatory Review
In April 2022, GenSight Biologics announced that the European Medicines Agency’s (EMA) Committee for Advanced Therapies (CAT) had granted the company a 6-month extension for responding to Day 120 questions in the regulatory review of LUMEVOQ®, GenSight’s gene therapy for the treatment of Leber Hereditary Optic Neuropathy (LHON).
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LUMEVOQ® Manufacturing Timeline
GenSight Biologics reported in April 2022 that the completion of the validation (PPQ) batches for LUMEVOQ®, the Company’s gene therapy for Laber Hereditary Optic Neuropathy treatment. The delay is required to undertake operational changes that will avoid the recurrence of problems with the most recent PPQ campaign. The campaign is expected to resume in the fourth quarter of 2022.
Designation
GenSight Biologics announced in September 2021 that its gene therapy LUMEVOQ® had been designated as a Promising Innovative Medicine (PIM) by the UK’s Medicines and Healthcare products Regulatory Agency (MHRA) for the treatment of vision loss caused by Leber Hereditary Optic Neuropathy (LHON) caused by a confirmed G11778A mutation in the ND4 mitochondrial gene.
Valoctocogene Roxaparvovec: BioMarin Pharmaceutical
Valoctocogene roxaparvovec is an investigational AAV5 gene therapy under regulatory consideration for the treatment of severe hemophilia A. It is presently in the preregistration stage of development. Hemophilia A is a bleeding condition caused by mutations in the F8 gene, which codes for the clotting protein Factor VIII. Patients with hemophilia A lack Factor VIII in their genetic makeup. For severe instances, frequent intravenous infusions of recombinant or plasma concentrated Factor VIII is usually used.
Valoctocogene roxaparvovec is a gene therapy that combines an adeno-associated virus 5 (AAV5) that codes for human Factor VIII with a human liver-specific promoter that stimulates translation in liver endothelial and sinusoidal cells, where Factor VIII is normally synthesized. The patient would be able to create adequate levels of physiologically active F8 gene by transfecting its functional form into liver cells.
Product Development Activities
Positive CHMP Opinion in Europe for Valoctocogene Roxaparvovec
BioMarin Pharmaceutical Inc. announced in June 2022 that the Committee for Medicinal Products for Human Use (CHMP) had adopted a positive opinion recommending Conditional Marketing Authorization (CMA) for valoctocogene roxaparvovec, an investigational gene therapy for adults with severe hemophilia A. The European Commission is likely to make a final clearance decision in Q3 2022, which will be consistent with the CHMP proposal.
Designation
In March 2021, the FDA designated valoctocogene roxaparvovec as a Regenerative Medicine Advanced Therapy (RMAT). RMAT is a streamlined program designed to speed the development and approval of regenerative medicine therapies, such as the valoctocogene roxaparvovec, that are intended to meet an unmet medical need in individuals suffering from severe diseases. The RMAT designation supplements the Company’s Breakthrough Therapy Designation, which it acquired in 2017.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
In addition to RMAT and Breakthrough Therapy Designation, BioMarin’s valoctocogene roxaparvovec has received Orphan Drug Designation by the FDA and EMA for the treatment of severe hemophilia A. The Orphan Drug Designation program aims to accelerate the assessment and development of medications that show promise for diagnosing and treating rare diseases or conditions.
Resubmission of a Biologics License Application to the U.S. Food and Drug Administration (FDA)
BioMarin announced in June 2022 that its intended timeframe for resubmitting a Biologics License Application to the U.S. Food and Drug Administration (FDA) for valoctocogene roxaparvovec, commonly known as roctavian, had been moved back to the end of September 2022 – the original target had been June.
NFS-01: Neurophth Biological Tech
NFS-01, a novel recombinant adeno-associated viral serotype 2 vector (rAAV2) containing a codon-optimized NADH-dehydrogenase subunit 4 (ND4) gene under the control of the cytomegalovirus promoter and enhancer, is being developed as an ophthalmic injection for the treatment of Leber hereditary optic neuropathy (LHON) associated with ND4 mutations.
Product Development Activities
The U.S. FDA granted NFS-01 (NR082, rAAV-ND4) an Orphan Drug Designation in September 2020, laying the groundwork for speeding the worldwide development of this therapy.
In April 2021, Neurophth reported that the Center for Drug Evaluation (CDE) of China’s National Medical Products Administration (NMPA) had accepted the Company’s Investigational New Drug (IND) application for NR082 for LHON patients with ND4 mutations. It is China’s first AAV2 gene therapy IND approval.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Moreover, Neurophth secured more than USD 60 million in round C investment in November 2021 to explore gene treatments for ophthalmic diseases. CMG-SDIC Capital, Sequoia Capital China, Sunshine Insurance, and China Merchant Bank International Capital are among the company’s major investors.
AAV-CNGA3: MeiraGTx
AAV-CNGA3, a gene therapy treatment meant to restore cone function, is delivered to the cone receptors at the back of the eye through subretinal injection. It was designed with a synthetic promoter associated with high gene expression to account for the higher quantity of protein required to restore cone function in achromatopsia (ACHM) patients with a CNGA3 gene mutation. MeiraGTx is conducting a Phase I/II clinical study of AAV-CNGA3 in children aged 3 to 15 years old with ACHM caused by CNGA3 gene mutations.
Product Development Activities
MeiraGTx announced in January 2021 that the US Food and Drug Administration (FDA) had given Fast Track designation to their AAV-CNGA3 gene therapy product candidate for the treatment of achromatopsia (ACHM) caused by CNGA3 gene mutations.
MeiraGTx and Janssen Pharmaceuticals began exploring AAV-CNGA3 in January 2019 as part of larger cooperation to develop and market gene therapies for the treatment of hereditary retinal disorders.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
MeiraGTx announced in August 2018 that the U.S. FDA’s Offices of Orphan Products Development and Pediatric Therapeutics had granted rare pediatric disease designation to the Company’s gene therapy product candidate AAV-CNGA3 for the treatment of patients with achromatopsia (ACHM) due to CNGA3 gene mutations.
What Lies Ahead?
Researchers have used gene and other nucleic acid transfer into cells for more than four decades as a research tool. The hunt for real gene treatments has been pushed by our increased understanding of the genetic components underlying particular diseases. New areas of study have gradually uncovered other possible avenues for gene delivery to be used therapeutically. Furthermore, the limits of current small molecule and protein therapy platforms have fueled the hunt for new therapeutic platforms that address such shortcomings. Gene treatments address all of these obstacles, particularly target accessibility. As a result, the hunt for safe and effective gene delivery methods has been a key emphasis in pharmaceutical research and development, and will ideally mark a paradigm change in how we approach disease-state intervention.Adeno associated virus was developed over 50 years ago and has since evolved into one of the most important gene delivery vectors in clinical research. AAV may become the vector of choice for most gene therapy applications due to its unique biology, simple structure, and lack of known disease correlations. Gene therapy using rAAV has been shown to be safe and well-tolerated in almost every clinical context where it has been employed. These studies, along with basic biology research, have revealed numerous aspects of this vector that might be used in future initiatives.

Downloads
Article in PDF