Changing Landscape of EBV-associated Diseases Treatment: Is T-cell Immunotherapy the Answer?
Feb 17, 2023
Table of Contents
Epstein–Barr virus (EBV), also known as human herpesvirus 4 (HHV-4), was discovered in the 1960s as an inescapable virus that infects almost 95% of the world’s population and causes nearly 1% of all cancers. Besides, the virus is a leading cause of infectious mononucleosis (IM) and is associated with different subtypes of lymphoma, sarcoma, and carcinomas, such as Hodgkin’s lymphoma, non-Hodgkin’s lymphoma, nasopharyngeal carcinoma, and gastric carcinoma. However, despite years of research and studies, the actual reason behind EBV-linked cancers remains unclear. In most people, the primary infection occurs in childhood and remains asymptomatic. However, nearly 50% of cases that occur during adolescence and early adulthood lead to infectious mononucleosis.
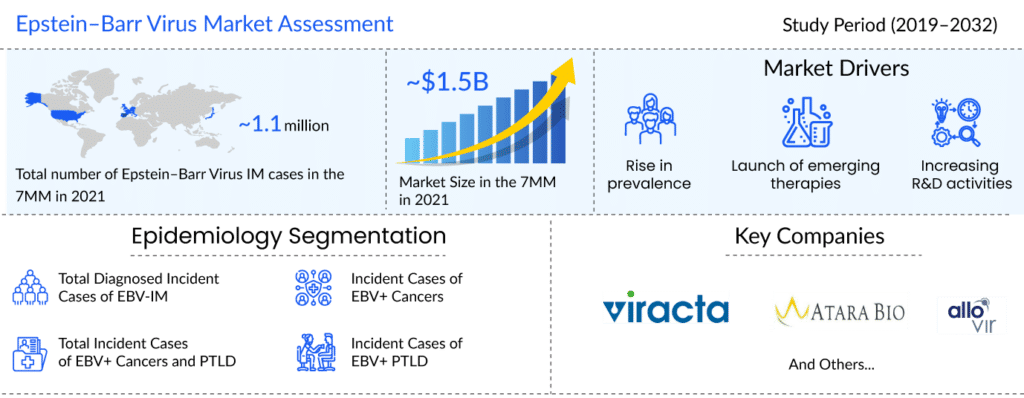
As per the latest “Epstein–Barr Virus Epidemiology” report by DelveInsight, in 2021, the total diagnosed incident cases of EBV-IM was more than 1.1 million in the 7MM. The United States had the highest number of incident cases of Epstein–Barr Virus in 2021. Assessments showed that in 2021, the number of cases of EBV+ HL was about 3.4K in the US, followed by EBV+ NPC with around 3.2K cases, EBV+ GC with more than 2.5K cases, and EBV+ BL with approximately 880 cases.
Downloads
Article in PDF
Recent Articles
- A New Addition to Existing Chimeric Antigen Receptor Therapies –Potential for a Game Changer in B...
- Systemic Lupus Erythematosus (SLE) – Market Is Expected to Grow with Upcoming Therapies
- Most Promising Oncological Drugs Expected to Launch in 2022
- Moderna’s mRESVIA(R) FDA Approval; Novartis Scemblix® Phase III Data; Kite’s Tecartus for R...
- CAR-T cell therapy market for Non-Hodgkin lymphoma: Future prospects, and untapped opportunities
Another life-threatening condition that prevails due to EBV is post-transplant lymphoproliferative disorder (PTLD), which is seen in patients following a solid organ transplant and allogeneic hematopoietic stem cell transplant (HSCT) due to the weakened immune system, leading to a reduction in T-cell response that is necessary to prevent EBV infection. Additionally, several reasons, including growing transplant activities, immunosuppressive drugs, improved diagnosis, and awareness, have led to a significant rise in PTLD patients over the last two decades. A recent longitudinal study has revealed that even multiple sclerosis links with EBV infection.
Current Management Strategies of EBV Treatment
The current EBV treatment landscape lacks approved therapies and focuses on symptomatic management of EBV infection. Different EBV drugs like antipyretics, analgesics, antivirals, and corticosteroids are continuously used to relieve symptomatic relief from infectious mononucleosis.
When taking care of PTLD or EBV infection in transplant patients, a reduction of immunosuppression is required to restore the host’s immunity. The American and British guidelines recommend adding rituximab monotherapy or rituximab in combination with cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP), depending on histology and clinical characteristics, although related toxicity and drug resistance have been reported with rituximab. Further, the management of EBV-related lymphomas works on similar lines, i.e., improvement in immune defects and antiretroviral therapy is considered appropriate for HIV-associated lymphomas. However, the present management is associated with relapse and refectory cases in EBV+ PTLD and lymphoma.
Vaccines – The Best Way to Keep Away EBV Infection
Being an omnipresent virus affecting most of the population, the most promising area of interest lies in developing an effective EBV vaccine. As a prophylactic agent, it aims at preventing the primary infection of EBV, ultimately terminating other secondary diseases associated with EBV infection. Despite years of continuous efforts and development, neither a therapeutic vaccine targeting EBV+ cancer nor EBV prophylactic vaccine has yet been approved. Nevertheless, attractive mechanisms and immunogens have been identified as potential options for vaccine development, for instance, EBV envelope proteins, including gH/gL, gB, and gp350. A few gp350-based vaccines include DNA vaccines, nanoparticle vaccines, epitope vaccines, and virus-like particles (VLPs). Other encouraging immunogens are latent and lytic proteins, including proteins expressed in various latency stages. However, the lack of an animal model to test EBV vaccines is major defiance in developing a vaccine. Nevertheless, several organizations and pharma giants work effortlessly to develop vaccines. Recently, ModernaTX Inc. has initiated Phase I for its EBV vaccine.
Promising Therapies Expected to Change the EBV Treatment Landscape
Several powerful and innovative immunotherapeutic strategies have emerged to treat PTLD and malignancies, especially those of hematological origin or resulting from a viral infection. Various checkpoint inhibitors and CAR-T cell therapies are in the line of development, along with small molecules to control the proliferation of B cells, and T cells, among others.
The absence of licensed drugs in the EBV treatment market offers a plethora of opportunities for the pharma players to take advantage of an untapped EBV treatment market. The future of EBV+ PTLD and cancer looks bright with promising therapies that would significantly impact the therapeutic pattern of EBV-related diseases. The key players that have identified potential candidates and successfully reached mid to late-stage development are Viracta Therapeutics, Atara Biotherapeutics, and AlloVir.
AlloVir’s Posoleucel is a multi-virus-specific T-cell therapy targeting five devastating viral pathogens: BK virus (BKV), Cytomegalovirus (CMV), Adenovirus (AdV), EBV, and human herpesvirus 6 (HHV-6). Additionally, tabelecleucel, investigated by Atara Biotherapeutics, is an off-the-shelf, allogeneic T-cell immunotherapy based on an allogeneic T-cell immunotherapy platform for treating patients with rituximab-refractory EBV+ PTLD. Viracta’s nanatinostat (VRx-3996) is a Class I-histone deacetylase (HDAC) inhibitor, a class of epigenetic-modifying drugs that affects the coiling of DNA around histones to change epigenetically-controlled expression patterns, being investigated in combination with valganciclovir for relapsed/refractory EBV+ lymphoma.
Recent Developments in the EBV Treatment Domain
- In December 2022, Atara Biotherapeutics announced updated interim analysis and safety results from its Phase III multicenter ALLELE study investigating tabelecleucel (tab-cel) to treat relapsed/refractory (R/R) EBV+ PTLD following SOT or HCT.
- In December 2022, AlloVir announced positive final results in phase II posoleucel multi-virus prevention study in an oral presentation at the 64th ASH Annual Meeting and Exposition.
- In October 2022, the CHMP adopted a positive opinion, recommending granting marketing authorization under exceptional circumstances for the medicinal product EBVALLO, intended to treat EBV+ PTLD.
- In September 2022, Viracta Therapeutics, announced that the European Commission (EC) had granted an Orphan Drug Designation (ODD) to nanatinostat and valganciclovir (nana-val), to treat peripheral T-cell lymphoma (PTCL).
Promising Future of the EBV Treatment Market
Over the last two decades, our understanding of the role of EBV infection in the pathogenesis and immune regulation of EBV-associated diseases has provided new lines of research to conceptualize a variety of novel prophylactic and therapeutic approaches to EBV-associated disease control. Some emerging approaches to EBV-related lymphomas include coupling agents that induce lytic viral replication with anti-herpesvirus agents or using small-molecule inhibitors that block signaling pathways that EBV constitutively activates.
DelveInsight estimates that with the approval of upcoming therapies, the EBV market is expected to reach approximately USD 3,000 million by 2032.
However, the pipeline for infectious mononucleosis and first-line treatment for EBV+ PTLD and cancer is limited, and more research is needed to explore treatment options for EBV-infected patients.
Moreover, EBV-specific cytotoxic T-cell infusions have been shown to be effective in EBV-PTLD, and research into expanding such adoptive immunotherapies to other EBV-related malignancies is ongoing. Furthermore, the rising prevalence and diagnosis would generate a lot of money in the future in the EBV treatment market.

FAQs
According to the Centers for Disease Control and Prevention (CDC), EBV, also known as human herpesvirus 4 (HHV-4), is a member of the herpes virus family. It is one of the most common human viruses. EBV can be found all over the world. Most people become infected with EBV at some point in their lives; EBV spreads most commonly through bodily fluids, primarily saliva.
With improved EBV laboratory tests, a faster and more accurate diagnosis could lead to a better prognosis and more effective treatment. In situ hybridization (ISH) of EBV-encoded RNA (EBER) in biopsy tissues remains the gold standard for detecting EBV in histopathological lesions. Methods for detecting EBV in various types of samples include the heterophile antibody test, immunofluorescence assays, enzyme immunoassays, Western blot, and polymerase chain reaction (PCR). However, detecting EBV viral load via PCR is gaining popularity in the diagnosis of EBV-associated diseases.
Prophylaxis, preventive therapy, and targeted therapy are examples of therapeutic strategies. As first-line therapy, rituximab, immunosuppression reduction, and EBV-specific cytotoxic T-cell therapy are recommended, with unselected donor lymphocyte infusions or chemotherapy as second-line options; other methods, including antiviral drugs, are discouraged.
Viracta Therapeutics, Atara Biotherapeutics, and AlloVir are the leading players in the global EBV treatment market. The launch of novel therapies in clinical development for disease management, as well as the development of basic research, clinical medicine, and new ideas related to the pathogenesis and clinical aspects of EBV-associated diseases, are expected to cause significant changes in the EBV treatment space during the study period (2019–2032).
Downloads
Article in PDF
Recent Articles
- Systemic Lupus Erythematosus (SLE) – Market Is Expected to Grow with Upcoming Therapies
- CAR-T Cell Therapy aka Miraculous Technology: Mapping the Market, Approved Therapies, Competitive...
- CAR-T in killing tumors; Amunix raises $73M; Autism findings in protein-targeted treatment
- Merck’s KEYTRUDA as Adjuvant Therapy for RCC Patients; BMS Receives Positive CHMP Opinion for CAR...
- CAR T-Cell Therapies in Non-Hodgkin’s Lymphoma Treatment: A Revolutionary Approach