The Evolving Landscape of Amyotrophic Lateral Sclerosis: A Fatal Disease!
Sep 24, 2024
Table of Contents
Amyotrophic Lateral Sclerosis (ALS) is a devastating neurodegenerative disease characterized by the progressive degeneration of motor neurons, leading to muscle weakness, paralysis, and ultimately, death. Over the past decade, a growing understanding of the disease’s underlying mechanisms has led to significant advancements in ALS therapy. New drugs, gene therapies, and precision medicine approaches are reshaping the treatment landscape of ALS, providing hope for better disease management.
Is ALS on the Rise?
Despite ALS being relatively rare, affecting 2-5 per 100,000 people worldwide, the question Is ALS on the rise? is gaining attention. While global prevalence has not significantly increased, improved diagnostic techniques, earlier detection, and greater awareness have led to a more accurate identification of ALS cases. Many researchers believe that enhanced surveillance and better tools for genetic testing are uncovering more cases than previously recognized, rather than a true rise in the disease’s incidence. However, with an aging global population, the burden of ALS may grow, as age is a major risk factor.
Downloads
Article in PDF
Recent Articles
- AstraZeneca’s Ultomiris; HebaBiz’s Siroquine; Pfizer’s XELJANZ; Bristol Myers S...
- Thrombolytic Sciences’ trial; NurOwn for ALS; Biosimilar receives approval; Trelegy Ellipta for t...
- Denali’s Impressive Research Portfolio
- Amylyx Raises $30M; QurAlis Announces License Agreement Deal With Lilly; COVID-19 Vaccine Updates...
- DelveInsight’s Central Nervous System Disorders based Gene Therapy Reports
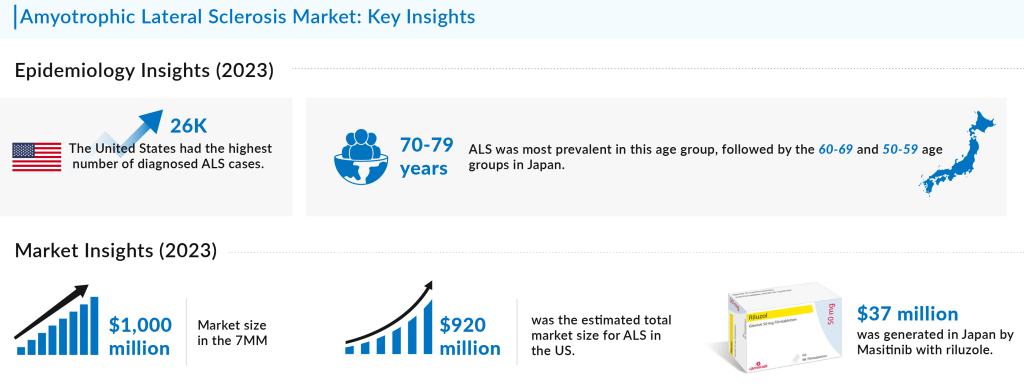
In 2023, the ALS incidence rate was highest in the US among the 7MM, with around 26,000 prevalent cases, a number expected to grow during the forecast period. Among EU4 and the UK, Germany had the highest diagnosed cases of ALS, while Spain recorded the lowest. The ALS survival rate remains low, especially in older populations, with the disease primarily manifesting in the spinal region, where 15,000 patients were reported, followed by bulbar onset cases. In Japan, ALS was most prevalent among individuals aged 70-79, followed by those in the 60-69 and 50-59 age groups.
Is ALS Fatal?
Amyotrophic Lateral Sclerosis (ALS), commonly known as Lou Gehrig’s disease, is indeed fatal. The disease is characterized by the progressive degeneration of motor neurons, which are essential for controlling voluntary muscle movements. As these neurons deteriorate, the brain loses its ability to initiate and manage muscle actions, leading to severe muscle weakness, paralysis, and eventually, respiratory failure. The relentless advancement of the disease, combined with the lack of a definitive cure or highly effective treatments, underscores why ALS is fatal. The average survival rate post-diagnosis is about 2 to 5 years, although some may live longer with extensive care. This rapid progression and impact on vital functions clarify why ALS is fatal and highlight the urgent need for more effective treatments and interventions.
Early Signs and Symptoms of ALS
Recognizing the early signs and symptoms of ALS is vital to improving patient outcomes. The initial symptoms can be subtle, including muscle twitching, cramping, and weakness in the limbs. These signs are often mistaken for other conditions, delaying diagnosis and limiting early intervention opportunities. Muscle stiffness, difficulty walking, and slurred speech can also appear in the early stages, signaling the onset of motor neuron damage. As the disease progresses, these symptoms intensify, affecting the ability to perform everyday activities.
Early diagnosis is crucial because the existing amyotrophic lateral sclerosis treatment options are most effective when initiated early. Catching ALS before significant nerve damage occurs could potentially slow the disease’s progression, though treatment remains palliative rather than curative. Physicians typically rely on a combination of electromyography (EMG), nerve conduction studies, and MRI scans to confirm ALS, along with genetic tests if a familial history is present. While new diagnostic tools are in development, timely recognition of symptoms remains the best path to effective disease management.
Treatment for ALS: Current Marketed Drugs
ALS remains a terminal condition, but treatment advancements have slowed disease progression and improved patient quality of life. The first significant breakthrough in ALS treatment came with the approval of Riluzole in 1995. Riluzole works by decreasing the release of glutamate, a neurotransmitter thought to play a role in ALS progression. While it doesn’t cure ALS, studies show that Riluzole can extend life expectancy by several months, typically adding about three to six months of survival. However, the Riluzole cost varies, and patients face high out-of-pocket expenses, depending on insurance coverage.
Another major drug is RADICAVA (edaravone), approved by the FDA in 2017. RADICAVA slows physical decline in ALS patients, particularly those in the early stages of the disease. Although it offers hope for extending the lives of those with ALS, RADICAVA’s cost is significant. The price for RADICAVA ORS (105 mg/5 mL) oral suspension is approximately $14,432 for a 50-milliliter supply, though the cost may vary depending on the pharmacy. These prices are for customers paying out of pocket and do not apply to those using insurance. The pricing information is based on using the Drugs.com discount card, which is accepted at many pharmacies across the U.S. In May 2022, an oral solution, RADICAVA ORS, was approved, providing a less invasive administration route than intravenous infusion. This breakthrough reflects ongoing efforts to develop more convenient and accessible treatment options for ALS patients, even as questions about affordability linger.
The Next Wave of ALS Treatments
The next generation of ALS treatments is targeting the root causes of neurodegeneration. Ransposon Therapeutics’ TPN-101 is one such promising candidate. TPN-101 is designed to target neuroinflammation, a significant driver of ALS progression. Preclinical studies have shown that reducing inflammation can preserve motor neuron function and potentially slow disease advancement. TPN-101 is currently in early-stage clinical trials, and if successful, it could offer an alternative to current therapies by addressing inflammation directly.
Similarly, Ionis Pharmaceuticals’ Ulefnersen (ION363), an antisense oligonucleotide therapy, is another emerging ALS therapy targeting a specific genetic cause. Ulefnersen aims to suppress the production of mutant FUS proteins, which are implicated in a subset of ALS cases. Antisense technology, like that used in Ulefnersen, offers the potential to silence harmful genes while leaving healthy versions intact, creating a more focused and less toxic approach to treatment. Both TPN-101 and Ulefnersen represent the next frontier of amyotrophic lateral sclerosis therapy, with the potential to offer patients more effective and personalized treatment options.
EXSERVAN’s FDA Approval: New Oral Solution
In November 2020, the FDA approved EXSERVAN, an oral film formulation of riluzole, offering ALS patients a new and more convenient method of drug administration. Unlike traditional riluzole, which is taken as a tablet, EXSERVAN dissolves on the tongue, making it easier to use for patients who have difficulty swallowing—a common symptom of ALS. This novel delivery method offers the same therapeutic benefits as riluzole, helping to slow the disease’s progression, but it improves accessibility for patients in later stages of ALS who may struggle with oral medication.
The EXSERVAN FDA approval highlights the importance of treatment accessibility in ALS care. As the disease progresses, swallowing becomes increasingly difficult, and alternative formulations like EXSERVAN provide a valuable solution. While the drug does not alter the course of ALS, innovations in drug delivery can significantly impact patient quality of life by making treatment easier to manage. This approval also underscores the broader trend in ALS drug development, where the focus is not only on new treatments but also on improving the delivery and accessibility of existing therapies.
RADICAVA Effectiveness and Ibudilast ALS Research
As one of the most notable ALS therapies, RADICAVA continues to be a focal point of clinical research. Studies have demonstrated that RADICAVA effectiveness is greatest in patients diagnosed early in their ALS progression. RADICAVA works by reducing oxidative stress, a factor believed to contribute to motor neuron death in ALS. While it does not halt the disease entirely, it can slow the decline in daily functioning, allowing patients to maintain independence for longer. However, the drug’s high cost and the need for regular intravenous infusions remain barriers for many patients, even with the introduction of RADICAVA ORS.
Meanwhile, Ibudilast has emerged as a potential treatment in ALS research. Originally developed as an anti-inflammatory drug for other neurological conditions, Ibudilast is now being studied in ALS for its ability to reduce neuroinflammation and protect motor neurons from further damage. Early-phase clinical trials have shown promising results, with some patients experiencing slower disease progression and improved motor function. If ongoing studies confirm these findings, Ibudilast could become a valuable addition to the arsenal of ALS therapies focused on modulating the immune system and protecting neurons from degeneration.
ALS Market Size and Future Outlook
Over the forecast period from 2020 to 2034, this market is expected to experience steady growth, driven by increased awareness, improved diagnostic capabilities, and the introduction of new therapies. The rising prevalence of ALS, advancements in medical technology, and ongoing research into disease-modifying treatments are key factors contributing to this market expansion. Additionally, the increase in R&D investments by pharmaceutical companies and the push for accelerated approvals from regulatory agencies are likely to boost the ALS market further.
The ALS market size is set to grow substantially in the coming years, driven by increased research investment, new drug for ALS, and growing awareness. In 2023, the total market size for Amyotrophic Lateral Sclerosis (ALS) in the United States was approximately USD 920 million. This growth is being fueled by the rising number of FDA-approved drugs, including innovative gene therapies like QALSODY, and the ongoing development of promising pipeline candidates such as Ransposon Therapeutics’ TPN-101 and Ionis Pharmaceuticals’ Ulefnersen (ION363). However, the high cost of current therapies, including Relyvrio, which ranges around $158,000 per year annually, continues to be a major concern. Moreover, Over the forecast period from 2020 to 2034, this market is expected to experience steady growth, driven by increased awareness, improved diagnostic capabilities, and the introduction of new therapies.
By 2034, the ALS market is anticipated to reach new heights due to emerging therapies that offer hope to patients with limited treatment options. However, one of the primary market drivers is the growing availability of innovative drugs and personalized approaches, especially as new therapeutic candidates continue to advance through clinical trials. The focus is shifting towards improving the quality of life for patients by slowing the disease’s progression, making ALS treatment a significant area of interest for biopharma companies.
However, affordability and access to these treatments will remain a key challenge. Governments and healthcare organizations must balance the need for cutting-edge therapies with the imperative to make. Aging populations, especially in developed countries, also contribute to an apparent rise in incidence, as ALS typically manifests in individuals aged 50-70.
Research into environmental factors such as exposure to toxins, military service, and professional athletics has shown potential links to ALS development, which could suggest a growing risk in specific demographics. The combination of these factors is contributing to the perception that ALS is becoming more prevalent, despite the stable incidence rates in most regions.
Existing Treatment Options for ALS
While there is no cure for ALS, treatment focuses on managing symptoms and improving the quality of life for patients. RILUTEK (riluzole) has long been the standard of care, showing modest benefits in extending survival, while RADICAVA (edaravone) offers another option, targeting oxidative stress to slow functional decline. These treatments, although essential, only provide limited efficacy, and the need for new therapies is crucial.
Symptomatic treatment includes the use of NEUDEXTA for managing pseudobulbar affect, along with physical therapy, assistive devices, and nutritional support. Advances in multidisciplinary care, such as respiratory management and palliative care, have significantly improved survival and quality of life for ALS patients, even though these approaches do not address the root cause of the disease.
EXSERVAN (riluzole oral film) received FDA approval for the treatment of ALS in November 2019, offering an alternative administration route for patients with dysphagia or difficulties swallowing pills. Its approval underscores the growing interest in improving the accessibility and delivery of existing therapies. TPN-101, developed by Ransposon Therapeutics, is another emerging therapy with promising results in slowing ALS progression by targeting TDP-43 aggregation, a key pathological hallmark of the disease.

Ionis Pharmaceuticals’ Ulefnersen (ION363), a promising investigational drug, is currently in clinical trials targeting mutations in the FUS gene, which is associated with aggressive ALS forms. These advancements are expanding the treatment options for ALS patients, especially those with genetic mutations, highlighting the ongoing effort to bring more precise and effective therapies to the market.
A milestone was achieved in ALS treatment when the FDA approved QALSODY (tofersen) in April 2023. QALSODY is a gene therapy specifically targeting patients with mutations in the SOD1 gene, which is responsible for 2-5% of all ALS cases. By inhibiting the production of abnormal SOD1 protein, QALSODY slows the progression of ALS in these patients, making it a groundbreaking therapy for a previously underserved population. This approval represents a key shift towards personalized medicine, offering more targeted and potentially more effective treatments for specific genetic subtypes of ALS.
QALSODY’s approval reflects broader progress in ALS drug development, as pharmaceutical companies and biotech firms focus on new drugs for amyotrophic lateral sclerosis that address underlying genetic mutations. This approach marks a paradigm shift from symptom management to disease modification, with researchers working to identify other genetic targets that contribute to ALS progression. While not all patients can benefit from QALSODY, its development represents a new chapter in ALS care, opening the door to future gene-based therapies and more individualized treatment plans.
The Role of Gene Therapy in ALS Treatment
Gene therapy has emerged as a groundbreaking area of ALS research, aiming to modify or silence faulty genes that contribute to the disease. ZOLGENSMA, a gene therapy for spinal muscular atrophy (SMA), has shown that altering genetic instructions can profoundly impact motor neuron diseases, providing hope for a similar approach in ALS. The introduction of tofersen by Biogen, targeting the SOD1 mutation in ALS, marks one of the first examples of gene silencing in the ALS treatment paradigm.
The potential of gene therapy extends beyond single-gene mutations, with efforts focused on more complex genetic interactions. Researchers are exploring ways to deliver gene-editing tools, like CRISPR, to the affected motor neurons, offering the possibility of halting or even reversing the disease progression. Although still in its early stages, gene therapy holds promise as a transformative treatment for ALS.
Emerging Therapies and Clinical Trials
Emerging therapies for ALS are offering new hope, with several candidates in the ALS pipeline showing promising results. Relyvrio, a combination drug developed by Amylyx Pharmaceuticals, was approved by the FDA in 2022. It aims to protect neurons from degeneration by targeting two distinct cellular pathways, marking a significant advance in ALS treatment. NurOwn, developed by BrainStorm Cell Therapeutics, is another notable therapy that utilizes mesenchymal stem cells to deliver neurotrophic factors directly to the brain and spinal cord, promoting motor neuron survival.
Clinical trials for these and other therapies, such as TPN-101 and Ulefnersen, continue to expand the horizon of ALS treatment. These emerging therapies not only target disease progression but also focus on improving the quality of life for patients by addressing the specific genetic and biological underpinnings of ALS. As more drugs advance through clinical trials, the ALS treatment landscape is set to undergo significant changes in the next decade.

Key Challenges in ALS Drug Development
Despite the promising developments in ALS treatment, significant challenges remain in drug development. ALS is a highly heterogeneous disease, with complex genetic, environmental, and cellular factors contributing to its onset and progression. This makes it difficult to develop one-size-fits-all treatments. The blood-brain barrier also poses a significant obstacle, preventing many drugs from reaching their intended targets in the central nervous system.
Moreover, the rapid progression of ALS complicates clinical trial design, as patients may experience significant functional decline during the study period, making it challenging to assess the long-term efficacy of new therapies. The need for biomarkers to predict disease progression and treatment response remains another crucial area of unmet need in ALS research, as these could help streamline drug development and improve patient outcomes.

Downloads
Article in PDF
Recent Articles
- Regeneron’s I-O Drug; Gene Therapy for Parkinson’s Disease; Yumanity and Merck Inks $...
- Spasticity
- Biogen terminates ALS Pact with Karyopharm; AbbVie’s Immunological Drug Skyrizi; NICE Backs Astel...
- Survodutide Phase II trial Shows Groundbreaking Results in Liver Disease; GSK Announces Positive ...
- Precigen’s PRGN-3006; Yescarta Approved as a First CAR T-cell Therapy for R/R LBCL; Biogen ...