Evolving Landscape of Multiple System Atrophy Treatment: Recent Developments and Future Directions
Jun 19, 2023
Table of Contents
Multiple system atrophy (MSA) is a rare disease that affects between 15,000 and 50,000 Americans, encompassing men and women of all races. Multiple system atrophy has an unknown cause. The great majority of cases are sporadic, meaning they happen at random. The peak onset of multiple system atrophy occurs between the ages of 55 and 60, with a range of 30 to over 90 years. The incidence of multiple system atrophy in the United States is estimated to be 0.6 cases per 100,000 persons per year in the general population, resulting in a current estimate of approximately 1,900 new cases each year.
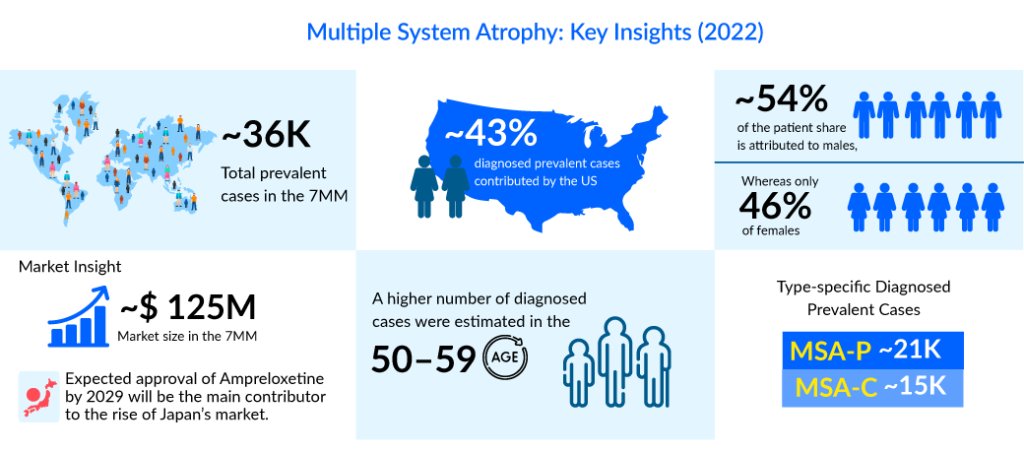
In the assessment done by DelveInsight, the estimated total diagnosed prevalent cases of multiple system atrophy in the 7MM were ~36K in 2022. The highest diagnosed prevalent cases of multiple system atrophy were found to be reported in the US compared to the other 7MM countries. As per DelveInsight’s estimates, the country alone accounts for ~43% of the total diagnosed prevalent cases among the 7MM countries.
Downloads
Article in PDF
Recent Articles
- Navigating the Healthcare Horizon: Odyssey of Mergers, Funding, and Acquisitions in 2024
- Voltage-Dependent T-Type Calcium Channel Blockers
- Bayer Phase III NSCLC Trial; D&D Pharmatech Gets FDA Nod for GLP-1R Agonist in Multiple Sclet...
- Byondis’s HER2-targeting ADC trastuzumab duocarmazine; AbbVie Migraine Drug Atogepant; Grünenthal...
- Parkinson’s Awareness Month 2019
There is approximately one live case of multiple system atrophy in the population for every 40 cases of Parkinson’s disease, but because MSA survival is shorter than that of Parkinson’s disease, around one new multiple system atrophy case appears each year for every 20 who present with Parkinson’s disease.

Neurodegenerative Connections: Similarities Between Multiple System Atrophy and Parkinson’s Disease
Multiple system atrophy and Parkinson’s disease are two neurodegenerative disorders that share several similarities in terms of clinical features and underlying mechanisms. While they are distinct conditions, they both belong to the group of synucleinopathies, characterized by the abnormal accumulation of the protein alpha-synuclein in the brain. Firstly, multiple system atrophy and Parkinson’s disease exhibit overlapping motor symptoms, such as bradykinesia, rigidity, and postural instability. Both conditions can lead to impaired coordination and difficulty with daily activities, impacting the patient’s quality of life.
Secondly, the underlying neuropathological changes in multiple system atrophy and Parkinson’s disease involve the degeneration of specific regions in the brain. In Parkinson’s disease, there is a loss of dopaminergic neurons in the substantia nigra, leading to a deficiency of dopamine in the basal ganglia. In multiple system atrophy, multiple regions are affected, including the basal ganglia, cerebellum, and autonomic centers. This widespread degeneration contributes to the diverse symptoms observed in multiple system atrophy, including autonomic dysfunction and cerebellar ataxia. Another similarity between multiple system atrophy and Parkinson’s disease is the presence of alpha-synuclein aggregates, known as Lewy bodies, within affected brain regions. These abnormal protein accumulations are thought to contribute to the progressive neuronal loss and subsequent clinical manifestations seen in both diseases.
Breaking Ground: Promising Multiple System Atrophy Treatment Approaches
In the approved market segment for multiple system atrophy treatment, NORTHERA (droxidopa) is the world’s first approved medication for symptomatic nOH. Dainippon Sumitomo Pharma (DSP) developed it first, and it was commercialized in Japan in 1989. In 2006, Chelsea Therapeutics International acquired the medication license from DSP. Chelsea received fast clearance for NORTHERA from the US FDA in February 2014 for symptomatic benefit in adult patients with nOH, and exclusivity expires in February 2021. nOH is found in around 66-90% of multiple system atrophy patients and is a leading source of disability and injury in the disease. There is currently no approved disease-modifying agent. The drug used to treat Parkinson’s disease, most notably levodopa (SINEMET), is also administered for multiple system atrophy patients. The efficacy of such drugs, however, varies substantially across affected individuals.
Other drugs used to treat Parkinson’s disease, in addition to levodopa, may be used to treat multiple system atrophy patients. These included dopamine agonists like ropinirole (Requip) and pramipexole (Mirapexin), which boost dopamine receptor activation in the brain. This aids the brain’s reception of dopamine messages. Midodrine hydrochloride (ProAmatine) has been used to treat low blood pressure, which has been linked to multiple system atrophy in certain cases. Low blood pressure can be treated using adrenergic medications such as ephedrine. Low blood pressure can also be treated with L-threo-dihydroxyphenylserine (L-DOPS or Lthreo-DOPS).
Midodrine is thought to be more physiologic and well-tolerated than fludrocortisone, and other medicines worth testing include indomethacin and intranasal desmopressin. The breadth of treatment options for orthostatic hypotension in multiple system atrophy provides clinicians with some hope of alleviating the misery of many patients. Multiple system atrophy covers the major market with off-label and generic treatments. Clonazepam, vitamin E, propanolol, baclofen, or amantadine have demonstrated superior efficacy. However, in an open-label experiment with MSA-C patients, buspirone (off-label) improved upper-limb ataxia. Depending on the severity of the other symptoms, drugs such as sildenafil (Viagra), tadalafil (Cialis), or vardenafil (Levitra) may be used to treat urinary and erectile dysfunction (ED) symptoms that are common in males with multiple system atrophy.
Promising Therapies and Future Scenarios for Multiple System Atrophy Treatment
Considering all the existing knowledge gaps and the void of effective neuroprotective treatment, some major pharma players are proactive with their early and mid-phase trial candidates in the pursuit of a breakthrough therapy to dominate the multiple system atrophy treatment market. H Lundbeck A/S (Lu AF82422), Theravance Biopharma (Ampreloxetine) (TD-9855), Brain Neurotherapy Bio, Inc (AAV2-GDNF gene therapy), Ionis Pharmaceuticals, Inc. (ION464), Alterity Therapeutics (ATH434), Enterin Inc (ENT-01). and others are the leading companies working in the segment with their lead assets in different multiple system atrophy clinical trials.
The human Monoclonal Antibody (mAb) Lu AF82422 targets the toxic alpha-synuclein protein, which is a pathogenic characteristic of multiple spinal atrophy. To eliminate these toxic proteins, the chemical operates similarly to the body’s natural immune system. Lu AF82422 may potentially reduce or stop the progression of MSA because it tackles the disease’s underlying biology, according to Lundbeck. The EMA granted Lu AF82422 ODD in April 2021, and the AMULET project has been approved by the FDA and is ready to recruit the first patient.
Theravance Biopharma’s ampreloxetine is a once-daily Norepinephrine Reuptake Inhibitor (NRI) in development for the treatment of patients with symptomatic neurogenic orthostatic hypotension (nOH). It binds to norepinephrine transporters with a high affinity. Ampreloxetine increases extracellular norepinephrine concentrations by inhibiting the activity of these transporters. Based on the clinical trial outcomes, SEQUOIA, REDWOOD, and OAK, TD-9855 does not now hold a strong position as an emerging player. However, based on the predicted safety and efficacy outcomes of the investigational drug from the updated Phase III clinical trial slated for 2023, this investigational medicine is projected to validate as a potent cure in MSA patients considering the symptomatic neurogenic nOH treatment.
Brain Neurotherapy Bio, Inc.’s AAV2-GDNF Gene Therapy began a Phase I trial to assess the safety and potential clinical benefit of AAV2-GDNF given to the putamen in participants with a possible or likely diagnosis of MSA. GDNF gene therapy enhances the survival and function of susceptible dopamine-producing brain cells “sick but not dead” in MSA-P by increasing levels of the Glial cell line-derived Neurotrophic Factor (GDNF), a naturally occurring growth factor in the brain. Using the brain’s cellular machinery, the suggested gene therapy offers continuous creation and release of GDNF, which supports dopaminergic neuronal health. It may be preferable to intermittent protein infusions of synthetic GDNF, in which brain levels may become subtherapeutic between infusions.
ENT-01 is an oral lab-made drug produced from squalamine, a substance identified in the liver and gall bladder of the dogfish shark. Preclinical studies suggest ENT-01’s efficacy in removing harmful alpha-synuclein aggregates and preventing their development within gut nerve cells, hence boosting cells’ normal signaling. Recently on 24 May 2023, Enterin Inc. announced the FDA’s acceptance of an investigator-sponsored Investigational New Drug (IND) application (166532) to treat a patient with prodromal multisystem atrophy (MSA) with ENT-01. Treatment will be continued continuously or until substantial adverse effects occur that necessitate drug withdrawal.
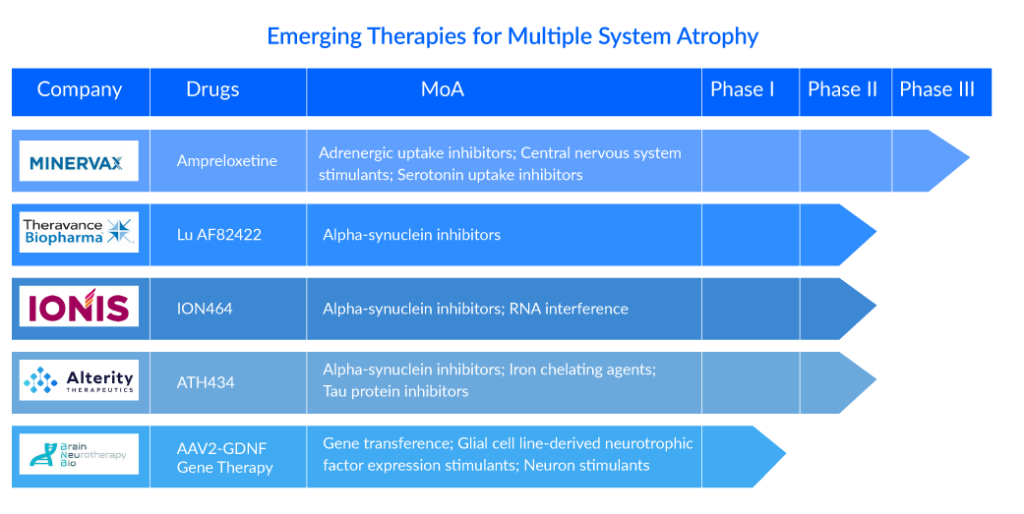
Alterity’s lead candidate, ATH434, is the first of a new generation of small molecules designed to block the aggregation of pathogenic proteins implicated in neurodegeneration. ATH434 decreases the aberrant accumulation of S and tau proteins in animal illness models by restoring normal iron balance in the brain. As a result, it has a high potential for treating various kinds of atypical Parkinsonism. Recently, Alterity Therapeutics announced that the first patient has been enrolled in new Phase II clinical trial investigating ATH434 in individuals with multiple system atrophy (MSA), a type of atypical parkinsonism. The open-label, biomarker ATH434-202 research (NCT05864365) at Vanderbilt University Medical Centre in Tennessee is enrolling 15 persons with advanced MSA and clinical indications of parkinsonism.
With the expected launch of these emerging therapies, the multiple system atrophy treatment market is expected to show positive growth in the forecast period in the 7MM. As per DelveInsight analysis, the total multiple system atrophy market size in the 7MM was approximately USD 125 million in 2022 and is projected to increase during the forecast period (2023–2032).
In recent years, there has been a better understanding of the neuropathological components of multiple system atrophy. In addition, research studies using effective MoAs to treat multiple system atrophy neuroprotective provide significant pharma companies with a plethora of exciting opportunities. As the current diagnostic consensus criteria strive for a step forward, the conclusive diagnosis of multiple system atrophy is opportunistic.
FAQs
Multiple system atrophy (MSA) is a neurodegenerative disease that causes symptoms that affect both the autonomic nervous system and mobility.
The slowness of movement, tremors, rigidity, and incoordination are the first indications and multiple system atrophy symptoms. The Parkinsonian type (MSA-P) with primary symptoms similar to Parkinson’s disease and the cerebellar type (MSA-C) with primary symptoms featuring ataxia are the most significant symptoms of examination.
Multiple system atrophy diagnosis is difficult, especially in the early stages, because many of the symptoms are similar to Parkinson’s disease. Autonomic testing, assessment of bladder function, and/or neuroimaging such as an MRI or PET scan are examples of diagnostic examinations. The diagnosis of multiple system atrophy is also dependent on a thorough medical history and neurological exams; the labels Possible, Probable, and Definite are used to describe different diagnostic phases in MSA cases.
There are currently no treatments available to slow the progression of multiple system atrophy’s neurodegenerations. There are drugs available to help with multiple system atrophy symptoms, such as Levodopa, Amantadine, Droxidopa, Anticholinergic agents, and various off-label therapy.

Downloads
Article in PDF
Recent Articles
- What Lies Ahead For Parkinson’s Disease Psychosis Treatment Market?
- FDA Approves GSK’s Arexvy for RSV; CHMP’s Opinion on Gilead’s Hepcludex® for HDV; FDA Clearance t...
- Parkinson’s Disease: How Close are we to a Cure?
- Decoding Parkinson’s Diagnosis with Gene Therapies and Prevention Insights
- Wireless Brain Sensors: Revolutionizing Neuroscience and Healthcare