Graft-Versus-Host Disease (GvHD) Treatment Market: A Moving Target For Therapies
Jun 23, 2023
Allogeneic HSCT is a mainstay for patients with different hematological malignancies, but it requires immunosuppressive strategies before and after HSCT. However, approximately half (50%) of the patients receiving allogeneic hematopoietic stem cell transplantation (HCT) from a human leukocyte antigen (HLA)-matched sibling experience acute GVHD. These rates are higher in unmatched donors. In the United States, around 25,000 hematopoietic stem cell transplants (HSCTs) are performed yearly, with ~40% being allogenic.
The treatment of GvHD involves using immunosuppressive drugs such as corticosteroids, calcineurin inhibitors, and monoclonal antibodies. Off-label therapies seem to be the flag bearer of the treatment landscape for GvHD sufferers. Patients are being treated with a combination of therapies as per their requirements and symptoms that might work for them or leave them struggling for advanced treatment.Till now, four drugs have been approved for GvHD treatment: ruxolitinib (JAKAFI), belumosudil (REZUROCK), and ibrutinib (IMBRUVICA), ORENCIA (abatacept).
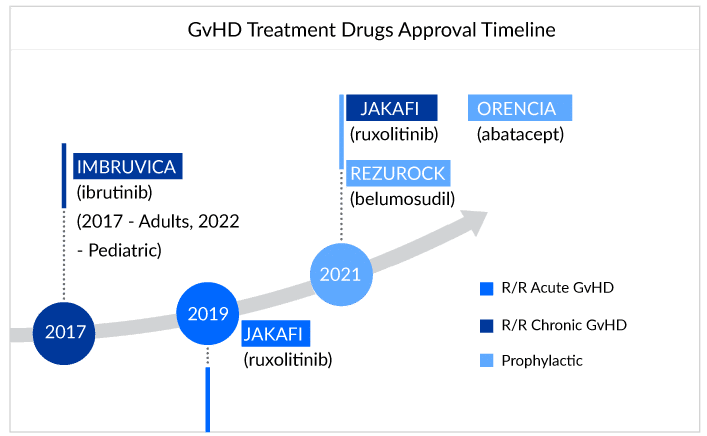
For refractory chronic GVHD patients, ruxolitinib is slowly establishing itself and now it is becoming the standard therapy in the second line, and below are the expert opinions that reflect the same. However, if patients do not respond to second-line therapy with ruxolitinib, experts rely on the belumosudil as Ibrutinib’s success still needs to be proven for chronic GvHD. The difference and the preference between JAK inhibitors and BTK inhibitors have been shown below:
Downloads
Article in PDF
Recent Articles
- Actinium Announces Phase III SIERRA Trial Results; FDA Approves Apellis’s Geographic Atrophy Drug...
- Incyte Corporation submits positive results of trials for its drug Ruxolitinib in GvHD
- Aktis’s Novel Targeted Alpha Radiopharmaceuticals; Koye Partners with Sonde Health; Novartis to S...
- FDA Approves Vertex’s ALYFTREK for Cystic Fibrosis; ZEPBOUND Gets FDA Approval for Obstructive Sl...
- Japan is transforming its Healthcare sector through Regenerative Medicine
The importance of JAK inhibitors in the GvHD treatment landscape is not hidden. JAK inhibitors reduce the severity of GvHD by inhibiting the JAK signaling pathway, which is the key regulator of GvHD pathogenesis.
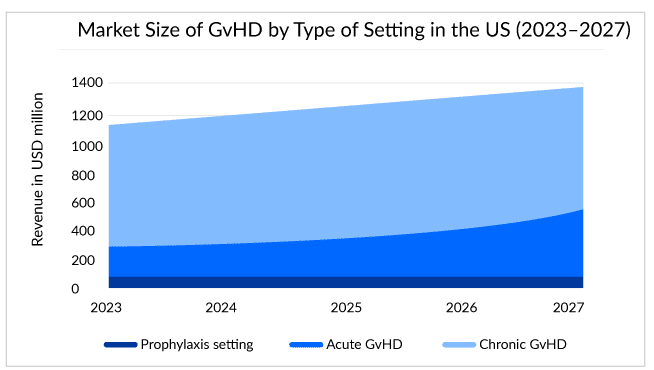
Estimates show that around 14,000 chronic GvHD and 5,000 acute GvHD patients live in the United States, and half fall under the steroid-refractory pool.
Recent label expansions and approval of JAKAFI (Incyte) and IMBRUVICA (AbbVie) for cGvHD certainly question whether the following approval can impact JAKAFI and IMBRUVICA market. Well, looking at prior off-label usage due to the inclusion of JAK inhibitors in NCCN guidelines, we assume a nominal effect for the companies. However, yes, this formal approval may help Incyte’s and AbbVie’s market to observe a boost in sales, as it paves the way for better access and reimbursement of drugs due to greater patient compliance. As per the estimates, GvHD-specific sales for JAKAFI could reach approximately USD 300 million by 2024.

The fast uptake of Kadmon’s REZUROCK (belumosudil) cannot be ignored in the GvHD treatment landscape. It works differently by rebalancing the immune system by blocking the ROCK2 pathway in the body, thus reducing inflammation and fibrosis due to chronic GVHD.
In just a year after the approval, REZUROCK holds a strong position with sales of approximately USD 220 million in the market, whereas JAKAFI and IMBRUVICA have been struggling to reach there for a long time.
REZUROCK has treated approximately 30% of the current potential patient pool worldwide. One survey showed the popularity of REZUROCK among doctors, where more than 70% of doctors showed their interest in prescribing REZUROCK (belumosudil) after the failure of ruxolitinib (JAKAFI).With similar costs associated with other branded drugs and, but with additional advantages over them, REZUROCK can emerge as a top influencer of the cGvHD treatment landscape and is estimated to generate peak sales of approximately USD 500 million. However, Kadmon may not be able to celebrate this for long as REZUROCK might observe a decline in sales soon after the patent expiration in 2026.
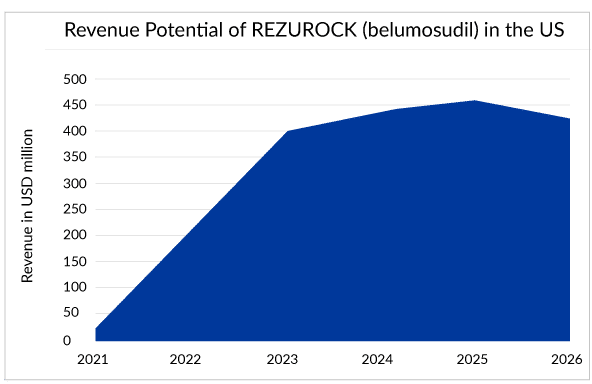
In December 2021, ORENCIA (abatacept) becomes the first and only therapy approved by the US FDA for prophylaxis. Since its initial approval in the year 2005, for the treatment of rheumatoid arthritis, ORENCIA has consistently gained use. It achieved blockbuster status in 2012. Then in its first full year following the psoriatic arthritis approval in 2018, the sales of the drug increased. Now, with all its approvals, for the treatment of rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, and the prophylaxis of acute graft versus host disease (aGVHD), the market of the drug has shown positive growth throughout the years.
With all the findings in clinical trials for GvHD, it was efficacious and well tolerated among patients, but it falls short for the pediatric pool as safety and effectiveness have not been established in pediatric patients
Needs that could drive the future direction of the GvHD treatment landscape
It is worth mentioning that there were similarities in the trial results of ruxolitinib (JAKAFI), belumosudil (REZUROCK), and ibrutinib (IMBRUVICA) and that most clinical responses were partial responses and not complete. Therefore, it becomes difficult to predict the agents’ responses in real-world settings as partial responses can change the life of the patients or show no difference. This raises many questions for the specialists about the appropriate agent usage, stage, intervening time, etc.
The GvHD treatment’s end result and primary objective are to fend off organ damage and improve patients’ quality of life. Hence, prognosis and early treatment are much needed for steroid-refractory patients.
NIH tools have contributed a lot in understanding GvHD pathophysiology better and assessing the severity. Now, to understand above discussed partial responses better, we need more similar kinds of tools with enhanced features, which can ultimately give convalescent outcomes. Hence, a lot of research is required in that palatinate.
In the chronic graft-versus-host disease treatment space, the real demand is for the segmentation of disease or biomarker development, which can help further in the stratification of this patient pool and understand their prognosis in a better way. However, unlike acute graft-versus-host disease, for chronic graft-versus-host disease biomarker development is way behind. Progression in this area can birth new targets or agents for intervention and evolution. A more personalized approach to chronic GvHD therapy can change the long-term outcomes and decrypt the patients with a dominant biologic pathway. Decrypting the biological pathway can help use the correct agents for the targeted treatment. With these, the chronic graft-versus-host disease space looks exciting, with three approved drugs in the last 5 years and likely more to come in the next few years.
Also, the potential of prevention in reducing the long-term impact on the occurrence and severity of chronic GvHD makes it an important component of GvHD treatment. Hence, building strategies for prevention or prophylaxis against chronic GvHD is the other important segment, and this can be a lucrative area for research in the next few years. However, looking at the real-world setting, experts need to focus and try to identify the target population at great risk of chronic GvHD after transplantation, which can improve the outcome and prevent organ damage. The upcoming pipeline for GvHD treatment seems clustered with many therapies for acute GvHD and chronic GvHD. Companies like Equillium/Biocon – EQ001 (itolizumab), Mesoblast/JCR Pharmaceuticals RYONCIL, Syndax Pharmaceutical – Axatilimab (SNDX-6352), and others are striving for approval.
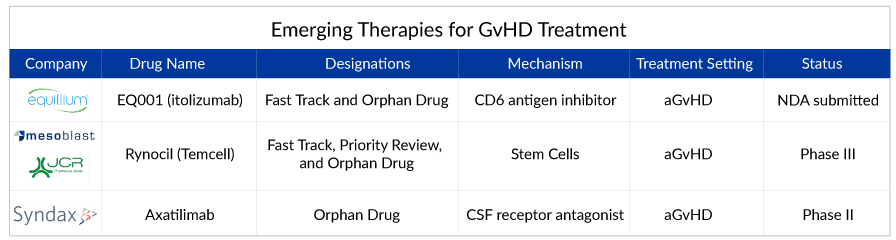
Aaxatilimab looks promising monoclonal antibody as researchers hope this novel pathway will be productive. This agent targets the CSF1R receptor, which is present in activated macrophages and can be useful in targeting the whole process of fibrosis. As per the studies, these activated macrophages are active in the fibrotic cascade.
Another candidate, RYONCIL (remestemcel-L), comprising culture-expanded mesenchymal stromal cells derived from the bone marrow of an unrelated donor, is approved in Japan for the treatment of aGvHD. However, the major shortcoming associated with RYONCIL (remestemcel-L) is being considered to be approved in pediatric patients only, leaving the huge adult patient pool to be easily picked by other drugs. On the contrary, Equillium’s drug candidate EQ001 also seems to be a potential drug candidate with a novel mechanism of action with additional advantages for the treatment of LGI aGvHD. With the approval of Equillium’s EQ001 (itolizumab) for treating acute GvHD, the drug might be in a pitched battle as the clinical trials of EQ001 seem promising.
In conclusion, the therapeutic candidates being studied might improve the quality of life and reduce the significant burden on the patients. Still, new approaches are required for better intervention. Potential strategies to improve GvHD therapies are to develop more targeted immunosuppressive drugs that specifically target the immune cells responsible for the GvHD while leaving the rest of the immune system intact. Another possibility is to use gene editing techniques to modify donor cells before transplantation to minimize the chances of GvHD. Additionally, researchers are exploring cell therapies that can reduce the severity associated with GvHD. While current therapies can effectively reduce the symptoms, they also present many side effects creating a need for effective, targeted, and safe therapies to treat this complication.
FAQs
Graft-versus-host disease (GvHD) is an immune condition that occurs after transplant procedures when immune cells (T cells) from the donor (known as the graft or graft cells) attack the recipient patient host’s tissues (healthy cells); the disease is a common side effect after an allogeneic bone marrow transplant (stem Cell Transplantation).
GvHD symptoms include rash, raised or discolored areas, skin thickening or tightening, jaundice, abdominal swelling, dry eyes or vision changes, dry mouth, white patches inside the mouth, pain or sensitivity to spicy foods, shortness of breath, difficulty swallowing, pain with swallowing, or weight loss, fatigue, muscle weakness, vaginal dryness or pain with intercourse, tightness in joints, and others.
The diagnosis of aGvHD is entirely based on clinical criteria that can be determined through a biopsy of one of the three target organs. Furthermore, laboratory data and/or imaging examinations are useful testing in the diagnosis of GvHD.
The GvHD treatment is based on medications that lower the body’s immunological response and the number of T cells. Immunosuppressive medicines are the current standard of care for GvHD. These include corticosteroid treatments as well as more advanced medications and procedures that suppress the immune response.

Downloads
Article in PDF
Recent Articles
- AstraZeneca’s Farxiga; Incyte’s Jakavi; FDA Fast Track Status to HM43239 for R/R AML; Idorsia’s I...
- FDA Approves ZURZUVAE for Postpartum Depression; Astellas Drug Acquired in $5.9B Deal Wins FDA Ap...
- Hematopoietic Stem Cell Transplantation
- Actinium Announces Phase III SIERRA Trial Results; FDA Approves Apellis’s Geographic Atrophy Drug...
- Fujifilm acquires global rights to Cynata’s novel therapy for GvHD; Themis raises EURO 40 m...



