Orchard’s OTL-200 to Enter US Metachromatic Leukodystrophy Treatment Space After EU
Sep 25, 2023
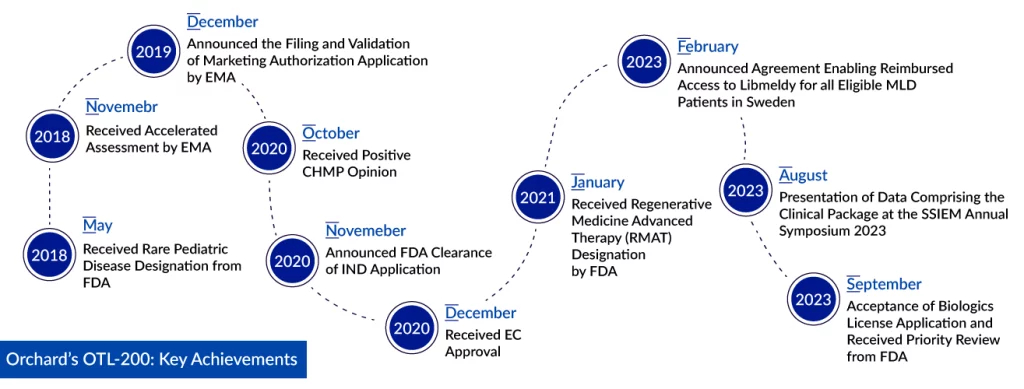
Orchard Therapeutics’ Biologics License Application for OTL-200, a gene therapy under investigation for metachromatic leukodystrophy treatment, has been accepted by the FDA. This rare disease treatment received approval in Europe back in 2020. Notably, the FDA has granted Orchard’s application Priority Review status, reducing the typical regulatory review timeline from 10 months to just six. We can expect the FDA’s decision on March 18, 2024.
“Today marks another significant milestone for patients and their families in the U.S. grappling with this devastating and heart-wrenching disease. They’ve endured an unimaginable journey, navigating the diagnostic challenges, receiving only supportive care options, and watching their children suffer”, said Dr. Bobby Gaspar, co-founder and CEO of Orchard Therapeutics. “We eagerly anticipate collaborating with the FDA during the review process. Given the urgency of treating children affected by MLD, we’re tirelessly preparing for a potential 2024 launch to make OTL-200 accessible to U.S. patients as swiftly as possible.”
Dr. Gaspar continued, “As we reflect on the remarkable progress we’ve accomplished over the past eight months since our Type B clinical meeting with the FDA, I want to extend my heartfelt thanks to the entire Orchard team, our invaluable clinical collaborators, external partners, and the MLD community. Your dedication has been instrumental in reaching this important milestone. While there are challenges ahead, it’s your collective efforts that are bringing us closer to realizing our shared goal of ending the suffering caused by severe genetic diseases like MLD.”
Downloads
Article in PDF
Recent Articles
- Metachromatic Leukodystrophy (MLD): A Rare Indication with great unmet medical need
- Bristol Myers Squibb’ Karuna Therapeutics Buyout; Orchard Therapeutics’ Lenmeldy FDA Approval; Ma...
- Overcoming the prevailing unmet needs in Beta-Thalassemia Market
- Orchard files for IPO; J&J puts money on RNA interference; Rgenix receives $40M; GSK gives a...
- DelveInsight’s Metabolic disorders based Gene Therapy Reports
Metachromatic leukodystrophy (MLD) is an exceptionally rare hereditary disorder, affecting roughly one in every 100,000 live births. This condition impacts the body’s metabolic system and is triggered by a pathogenic mutation in the arylsulfatase-A (ARSA) gene. Under normal circumstances, this gene encodes an enzyme responsible for breaking down sulfatides, a type of fatty substance. However, in individuals with MLD, this genetic abnormality results in the accumulation of sulfatides in various organs, including the brain, kidneys, and spleen. This buildup leads to a range of symptoms, including motor and cognitive impairments, behavioral issues, seizures, and spasticity. As the disease advances, MLD patients progressively lose their ability to move, speak, eat, and see, eventually leading to a fatal outcome. As per DelveInsight analysis, the prevalent cases of metachromatic leukodystrophy is expected to increase in the 7MM during the forecast year, with an improvement in treatment pattern as well.
OTL-200, also referred to as atidarsagene autotemcel, tackles the root cause of MLD by introducing functional copies of the ARSA gene. This experimental treatment involves the use of hematopoietic stem cells that have undergone genetic modifications to carry the human ARSA gene. Orchard Therapeutics’ received full (standard) market authorization for Libmeldy, a lentiviral vector-based gene therapy for the treatment of early onset MLD, in December 2020. It has been utilized in a minimum of six countries to date. Furthermore, the FDA has bestowed the candidate with both Rare Pediatric Disease and Regenerative Medicine Advanced Therapy designations.
The submission of the Biologics License Application (BLA) for OTL-200 draws upon a dataset gathered from 39 pediatric patients diagnosed with early-onset metachromatic leukodystrophy. These patients were enrolled in two prospective non-randomized clinical studies, involving 30 individuals, or they received treatment under expanded access frameworks, totaling 9 patients. These individuals underwent OTL-200 treatment and were compared with 49 untreated MLD patients, whose natural disease progression was tracked. All treated patients received OTL-200 and were closely monitored at Ospedale San Raffaele in Milan, Italy.
In the clinical trials, OTL-200 treatment demonstrated a remarkable preservation of motor function and cognitive development in the majority of patients when contrasted with the natural history of the disease, with a follow-up period extending up to 12 years (median 6.76 years). With a cumulative follow-up exceeding 250 patient years, OTL-200 treatment exhibited a favorable safety profile, with no occurrence of serious adverse events or treatment-related deaths. The majority of adverse events observed were linked to the busulfan conditioning regimen or the underlying disease. The comprehensive clinical results from the BLA dataset were recently presented at the 2023 Society for the Study of Inborn Errors of Metabolism (SSIEM) Annual Symposium in Jerusalem.
This BLA submission has accelerated the competition in the metachromatic leukodystrophy treatment space. Several key companies such as Takeda, Talaris Therapeutics, Magenta Therapeutics, and others are actively working with their lead assets and have sped up the process to give competition to Orchard’s gene therapy. Takeda with its candidate TAK-611 is the only company to be in the race, the rest all are evaluating their products in the early and mid stages of development.
Shire acquired Zymenex’s lead product Metazym (enzyme replacement therapy for MLD) and named it as SHP611, which was then acquired by Takeda. The drug is now known as TAK-611. It is a recombinant human arylsulfatase A (injection). The drug can enter the brain parenchyma including trans ependymal movement from ventricles through ependymal cells via gap junctions and perivascular and perivascular (glymphatic) movement via the pia/glia limitans. In October 2020, the results of phase I/II were published in the journal Molecular Genetics and Metabolism, which stated that intrathecal rhASA was generally well tolerated at doses up to 100 mg EOW. No rhASA-related serious adverse events (SAEs) were observed; 25% of patients experienced an SAE related to the intrathecal device or drug delivery method. These preliminary data supported further development of rhASA as a therapy for patients with MLD. Currently, it is being investigated in a global phase II clinical developmental trial for late-infantile MLD. The company expects the therapy to be approved by 2024.
As gene therapies are quite costly and far more expensive than enzyme replacement therapies, so it will give Takeda an upper hand over Orchard. Both therapies are expected to be launch in next year, so it will be great to watch the battle between these two giants. It will be interesting to see which therapy will grab the major chunk of the metachromatic leukodystrophy treatment market and who will see the face of defeat. Nonetheless, the pipeline of metachromatic leukodystrophy treatment possesses few potential key players, but still, the dynamics of the metachromatic leukodystrophy treatment market are anticipated to change in the coming years. This is mainly due to the improvement in the research and development activities so that the metachromatic leukodystrophy treatment market will comprise of efficient treatment options in the coming years.

Downloads
Article in PDF
Recent Articles
- DelveInsight’s Metabolic disorders based Gene Therapy Reports
- Bristol Myers Squibb’ Karuna Therapeutics Buyout; Orchard Therapeutics’ Lenmeldy FDA Approval; Ma...
- Gilead remunerates; Roche taps Halozyme’s drug delivery tech; Orchard Therapeutics prices its IPO
- Overcoming the prevailing unmet needs in Beta-Thalassemia Market
- Biogen’s Aduhelm; FDA Approves Sanofi’s Enjaymo; NHS & Orchard Signs a Deal; Bri...



