Unraveling the Complexities of Dermatomyositis Treatment: A Comprehensive Review and Future Perspectives
May 27, 2025
Table of Contents
Dermatomyositis, characterized by muscle weakness and skin symptoms, is a condition linked to increased morbidity and mortality. It markedly affects the quality of life for individuals afflicted, as well as the well-being of their families. According to DelveInsight’s estimates, in 2023, there were nearly 72K diagnosed prevalent cases of dermatomyositis in the seven major markets (the US, Germany, France, Italy, Spain, the UK, and Japan), with approximately 91% of cases occurring in adults and 9% in juveniles.
Understanding the Disease
Dermatomyositis is a rare autoimmune disorder associated with muscle inflammation (myositis) and skin inflammation (dermatitis). It falls under the classification of idiopathic inflammatory myopathies (IIM), a group comprising various connective tissue disorders characterized by the progressive weakening of muscles. The signs and symptoms of dermatomyositis may manifest suddenly or evolve gradually over time. The most common dermatomyositis symptoms include skin rash, muscle pain and weakness, fatigue, and ulcers. The precise cause of dermatomyositis remains unknown, although it is believed to stem from a combination of genetic predisposition and environmental stimuli.
Downloads
Click Here To Get the Article in PDF

Dermatomyositis affects people of all ages and genders, but is more common among women. The disease can occur in childhood and is usually referred to as juvenile dermatomyositis (JDM). While both adult and juvenile dermatomyositis conditions exhibit characteristic signs like distinctive skin rashes and muscle inflammation, they are heterogeneous with various additional features and complications. For example, vasculopathy is more prevalent in juvenile cases than adult dermatomyositis. Similarly, although children face an elevated risk of calcinosis and ulceration, their long-term outlook is generally more favorable.

Current Diagnostic and Dermatomyositis Treatment Landscape
Identifying the disease poses a challenge because of its resemblance in presentation to other forms of myositis. Accurate diagnosis involves a blend of clinical assessment, identification of distinctive physical indicators, specific laboratory tests, imaging examinations, and, in certain instances, muscle biopsies. Diagnostic criteria have been formulated, taking into account classification, pathological features, and symptoms of the disease.
In 1975, Bohan and Peter first suggested a set of five criteria to aid in the diagnosis and classification of dermatomyositis, which was accepted worldwide. Later on, other diagnostic criteria, including the European League Against Rheumatism/American College of Rheumatology (EULAR/ACR) guideline for adults and juvenile IIM, published in 2017, emerged.
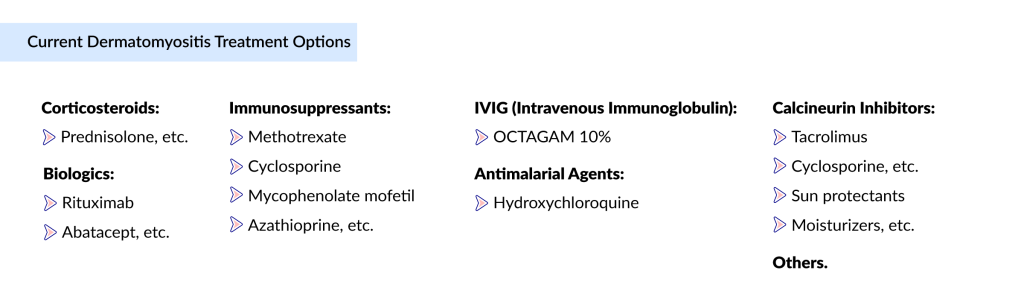
There is no cure for dermatomyositis, but medicines can minimize symptoms, reduce inflammation and vasculitis, and enhance the patient’s quality of life. The goals of managing dermatomyositis are focused on treating muscle weakness and skin disease and addressing any other underlying complications, including cardiac, pulmonary, gastrointestinal, joint, and malignancies. The recent approval of OCTAGAM and several management guidelines, including the British Society for Rheumatology guideline, the Japanese Society of Rheumatology guideline, and others, have simplified the treatment landscape for dermatomyositis. The first-line treatment for dermatomyositis includes the use of systemic glucocorticoids with or without immunosuppressants. Other dermatomyositis medications, including antimalarial drugs, antibiotics, and topical ointments, are given individually or in combination to eliminate symptoms. Further, rituximab, mycophenolate mofetil, calcineurin inhibitors, IVIG, and cyclophosphamide are preferred for resistant cases, patients unresponsive to initial treatment.
Medical therapy for skin disease includes topical and systemic medications. Topical agents include corticosteroids and calcineurin inhibitors. Most patients require systemic drugs to control skin disease, including hydroxychloroquine and methotrexate. Besides, nonpharmacological management options such as diet and physiotherapy, plasmapheresis, sun-protective measures, extracorporeal photochemotherapy, and total body irradiation, are employed as adjunctive treatment methods in cases of therapy-resistant dermatomyositis. While surgery is not a common approach for dermatomyositis, gastrotomy may be considered beneficial for patients experiencing severe esophageal dysfunction, and surgical removal of calcinotic nodules, if present, may also be performed.
According to DelveInsight, the market size for immunoglobulins in dermatomyositis was approximately USD 119 million in 2023, making it the leading treatment segment ahead of biologics, immunosuppressants, and corticosteroids. The overall dermatomyositis market size in the 7MM reached around USD 187 million in 2023 and is expected to grow at a CAGR of 16.8%, driven by increasing disease awareness, improved diagnostics, and the launch of emerging therapies.
Ongoing Challenges and Unresolved Obstacles in Dermatomyositis Treatment
To ensure proper care and enhance the well-being of individuals diagnosed with dermatomyositis, it is essential to acknowledge and address various deficiencies to fill the gaps. Some hurdles and challenges in the development and appropriate management of dermatomyositis include: Ensuring optimal care and improving the well-being of individuals diagnosed with dermatomyositis requires addressing various gaps and overcoming dermatomyositis treatment challenges. Some hurdles include:
Heterogeneity in the Pathophysiology
Although adults and JDM share the hallmark features of dermatomyositis, they are heterogeneous disorders with various additional disease features and complications. The occurrence of significant clinical aspects like calcinosis, interstitial lung disease, and malignancy significantly differs between adult and juvenile cases. Consequently, comparing disease outcomes between juvenile and adult myositis is challenging due to the absence of standardized outcome measures.
Complexity in Epidemiological Data
Dermatomyositis is rare, and its epidemiology studies on prevalence or occurrence are scarce. The complex pathophysiology of dermatomyositis, coupled with overlapping symptoms with other idiopathic inflammatory myopathies (IIMs), adds intricacy to its epidemiological study. There is a need for new studies to acquire a more comprehensive understanding of the disease epidemiology, encompassing its various types, degrees of severity, and variations across different geographical regions.
Early Detection and Diagnostic Gaps
Early detection of dermatomyositis is crucial for initiating appropriate patient management. Myositis mimics are prevalent, and their shared features with idiopathic inflammatory myopathies (IIMs) present a diagnostic challenge, often leading to misdiagnosis and delays in dermatomyositis treatment. Such delays can result in complications such as lipodystrophy, loss of function and muscle mass, calcinosis, joint contractures, and involvement of extra-muscular tissues.
Scarcity of Effective Therapies
OCTAGAM 10% is the only approved drug for dermatomyositis treatment. The use of OCTAGAM is restricted due to potential side effects such as flushing, headache, dizziness, chills, etc. Despite standard off-label therapies, patients frequently endure flares, leading to disability and reduced productivity. Additionally, these off-label medications, including corticosteroids, are linked to various side effects and adverse events, such as osteoporosis, heightened susceptibility to infections, cushingoid features, and more. There is a pressing need for safer and more effective treatment options to enhance patient outcomes.
Challenges in the Animal Model
Animal models serve as crucial tools for exploring new agents and understanding the mechanisms of various diseases. Despite various animal models providing insights into the underlying mechanisms of idiopathic inflammatory myopathies (IIM), none of these models fully replicate the clinical and pathological characteristics of human IIM, specifically dermatomyositis, resulting in limited resemblance.
Promising Triumphs on the Horizon for Dermatomyositis Treatment
In the last two decades, a substantial advancement in research has yielded new insights into the genetic risk factors and mechanisms underlying dermatomyositis, paving the way for promising therapeutic approaches to address the urgent need for effective dermatomyositis treatments.
Several dermatomyositis therapies are progressing through late-stage clinical development, including Priovant Therapeutics/Pfizer’s Brepocitinib, Kezar Life Sciences’ KZR-616 (zetomipzomib), argenx’s EFG PH20 (efgartigimod + PH20 hyaluronidase), Pfizer’s PF-06823859 (dazukibart), CSL Behring’s HIZENTRA, Horizon Therapeutics’ Daxdilimab (HZN-7734), and PAEAN Biotechnology’s PN-101. These dermatomyositis therapies, many of which are already approved for other autoimmune conditions, are currently in Phase II/III and Phase III trials. Their favorable safety profiles and demonstrated efficacy in related indications support their potential to significantly improve outcomes in dermatomyositis.
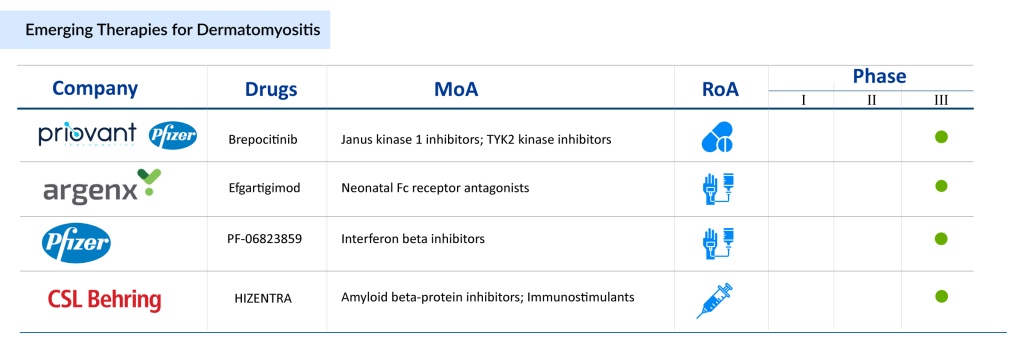
Brepocitinib: Tyrosine kinase (TYK2) and Janus kinases1 (JAK1) inhibitor
- Numerous case reports on tofacitinib in dermatomyositis provide compelling evidence supporting the potential efficacy of brepocitinib
- The pathobiology of dermatomyositis is influenced by dysregulation in cytokines, with signaling mediated by TYK2 and JAK1, particularly Type I interferon. This suggests that brepocitinib may exhibit greater efficacy than tofacitinib
- Comparative analyses between brepocitinib (30 mg daily) and tofacitinib in various autoimmune conditions indicate that dosing brepocitinib at 30 mg daily could yield clinically meaningful efficacy in dermatomyositis
- Delveinsight analysts anticipate that the drug will enter the US market by 2026 and reach its peak in the seventh year
PF-06823859: Interferon beta one fibroblast (IFNβ1) blocker
- In the Phase I trial, PF-06823859 demonstrated an acceptable safety, tolerability, and PK profile that supports clinical development for treating disorders associated with increased interferon β levels, such as dermatomyositis or systemic lupus erythematosus.
- In the Phase II trial, the drug improved clinical parameters like Manual Muscle Testing-8, Physician Global Assessment, patient-reported outcomes, and Health Assessment Questionnaire Disability Index in patients with muscle-predominant refractory moderate-to-severe dermatomyositis.
- As per Delveinsight, the drug is anticipated to enter EU4 (Germany, France, Italy, and Spain) and the UK market by 2027 and will attain its peak in the seventh year.
In summary, dermatomyositis is a rare and complex condition marked by limited public awareness. Diagnosis poses challenges, often resulting in significant delays in obtaining a definitive diagnosis for dermatomyositis patients. Additionally, existing biotherapies do not provide a cure. The visible skin manifestations, coupled with the complexities of the disease, may contribute to psychosocial and emotional stress in individuals with dermatomyositis. As a result, there is an urgent call for extensive research and development to tackle the existing challenges in managing dermatomyositis.

Frequently Asked Questions
Dermatomyositis is a rare autoimmune disease characterized by inflammation of muscle (myositis) and skin (dermatitis). In 2023, there were approximately 72,000 diagnosed cases in the seven major markets (US, Germany, France, Italy, Spain, UK, Japan). About 91% of cases are in adults, 9% in juveniles.
There is no cure, but treatment aims to reduce inflammation, skin complications, and muscle weakness. First-line treatments include systemic glucocorticoids with or without immunosuppressants. Other therapies used are antimalarials, antibiotics, topical steroids or calcineurin inhibitors, and for resistant cases, agents like rituximab, mycophenolate mofetil, IVIG, calcineurin inhibitors, or cyclophosphamide.
Several promising therapies are in development for dermatomyositis, with many advancing into late-stage clinical trials. Key candidates include Brepocitinib, a TYK2/JAK1 inhibitor showing potential in modulating immune pathways, and PF-06823859, an IFNβ1 blocker targeting inflammatory processes. KZR-616 (zetomipzomib) is another notable agent, designed to regulate immune responses by selectively modulating protein degradation. In addition, Efgartigimod combined with PH20 hyaluronidase is being evaluated to enhance antibody clearance, while HIZENTRA, Daxdilimab (HZN-7734), and PN-101 are emerging as potential options to expand treatment choices for patients. These investigational therapies highlight the growing focus on targeted and innovative approaches beyond conventional immunosuppressants.
Key obstacles include disease heterogeneity (adult vs juvenile forms have different features), diagnostic delays, lack of standardized outcome measures, and limited effective therapies. Also, animal models do not fully mimic human disease, which complicates preclinical research. There is also the issue of side effects from existing treatments (e.g. steroids).
The market size in 2023 was about USD 187 million in the 7 major markets, with immunoglobulins (e.g. IVIG) being the largest segment (~USD 119 million). The market is projected to grow at a CAGR of ~16.8%, driven by rising awareness, better diagnostics, and the arrival of new therapies.
Downloads
Article in PDF