Wainua – The Only Approved Medicine for hATTR-PN Treatment
Jan 05, 2024
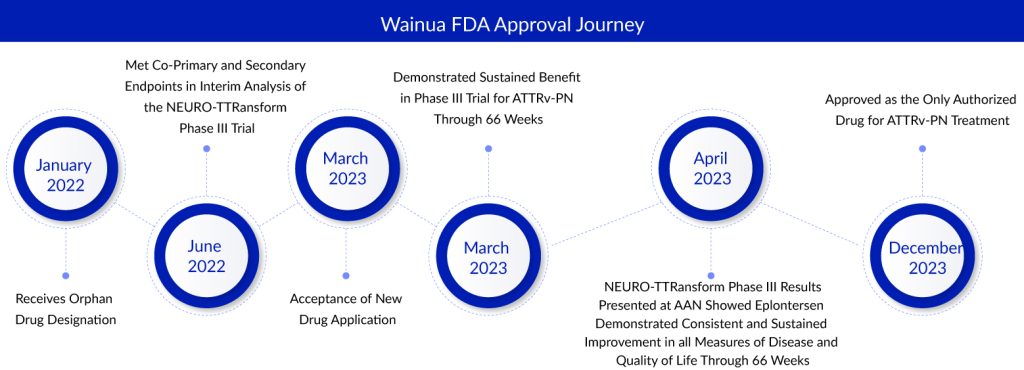
Ionis Pharmaceuticals and AstraZeneca concluded the year 2023 on a positive note as they received the eagerly awaited approval from the FDA for their transthyretin amyloidosis (ATTR) medication, Wainua. In particular, the agency approved the use of the ligand-conjugated antisense oligonucleotide (LICA) medication for addressing polyneuropathy in adults diagnosed with hereditary transthyretin-mediated amyloidosis (ATTRv-PN), a rare and frequently lethal condition impacting approximately 40,000 individuals worldwide. Wainua stands as the only authorized medication for treating ATTRv-PN, and it is designed for self-administration through an auto-injector.
ATTR cardiomyopathy and polyneuropathy represent progressive systemic conditions arising from either the aging process or genetic mutations. These conditions lead to the misfolding of TTR proteins, causing their aggregation into amyloid fibrils within the cardiac myocardium and peripheral nerves, respectively. In individuals with ATTR, whether hereditary or wild type (non-hereditary), TTR proteins accumulate as fibrils in various tissues, including the peripheral nerves, heart, gastrointestinal system, eyes, kidneys, central nervous system, thyroid, and bone marrow. The presence of these TTR fibrils disrupts the normal functions of these tissues. As the accumulation of TTR protein fibrils intensifies, it induces more tissue damage, exacerbating the disease and ultimately resulting in diminished quality of life and eventual mortality.
Dr. Michael J. Polydefkis, a Neurology Professor at Johns Hopkins University School of Medicine and a participant in the NEURO-TTRansform study, expressed that individuals affected by hereditary transthyretin-mediated amyloid polyneuropathy face challenges in fully enjoying their lives due to the persistent, advancing, and incapacitating impact of the disease. The approval of Wainua signifies significant progress in treatment, assisting those coping with transthyretin-mediated amyloid polyneuropathy in managing the effects of the condition.
Downloads
Article in PDF
Recent Articles
- Will The Burgeoning Gene Therapies Make a Difference in Dystrophic Epidermolysis Bullosa Patients...
- Cost ineffective Orphan Drugs limit the access
- Gaucher disease Market Analysis: Rich Pipeline Drives the Market Ahead
- Top 12 Most Expensive Drugs in the US Healthcare Market
- Celiac vaccine trial; New discovery in Nicotine; Nabriva lines up FDA filing; Rare Disease drug d...
The US Food and Drug Administration (FDA) granted approval based on a favorable 35-week interim analysis from the NEURO-TTRansform Phase III trial. This analysis revealed consistent and enduring benefits for patients treated with Wainua, as evidenced by positive outcomes on the co-primary measures of serum transthyretin (TTR) concentration and neuropathy impairment assessed through the modified Neuropathy Impairment Score +7 (mNIS+7). Additionally, the treatment demonstrated positive effects on a key secondary measure, namely, the quality of life (QoL) as assessed by the Norfolk Quality of Life Questionnaire-Diabetic Neuropathy (Norfolk QoL-DN). The encouraging findings from the NEURO-TTRansform Phase III trial, showcasing the efficacy of Wainua across different time points (35, 66, and 85 weeks), were published in The Journal of the American Medical Association (JAMA).
Ruud Dobber, the Executive Vice-President of AstraZeneca’s BioPharmaceuticals Business Unit, emphasized the pressing medical requirement for innovative treatments for individuals affected by hereditary transthyretin-mediated amyloid polyneuropathy. He highlighted the significance of the approval of Wainua in the United States, describing it as a fresh therapeutic choice that ensures a continual and significant decrease in serum TTR concentration from the baseline. Moreover, he underscored its ability to halt the progression of the disease and enhance the quality of life for those grappling with this incapacitating condition.
Isabelle Lousada, President and CEO of the Amyloidosis Research Consortium, emphasized that individuals dealing with hereditary transthyretin-mediated amyloid polyneuropathy, as well as other amyloidosis forms, frequently face misdiagnosis due to symptom similarities with other conditions. The process of obtaining a precise diagnosis is often a prolonged and challenging journey. It is crucial to promptly and accurately diagnose not just for the affected individual but also for their families and loved ones. Lousada expressed enthusiasm for the emergence of new innovations and increased initiatives to raise awareness in an area that has historically been overlooked or neglected.
Importantly, Wainua is available in an auto-injector format, allowing patients to self-administer the treatment monthly. This provides a significant advantage in terms of convenience compared to competing treatments like Alnylam’s Onpattro, which requires subcutaneous administration at treatment centers. Combining Ionis’ extensive knowledge in amyloidosis and rare diseases with AstraZeneca’s expertise in cardiovascular diseases and global presence allows the collaboration to impact a wide range of patients. AstraZeneca sees the approval as the beginning of an exhilarating venture into the field of amyloidosis.
The Wainua collaboration is not concluding at this point. The next step for the medication involves a scheduled attempt to broaden its approved uses to include transthyretin amyloid cardiomyopathy (ATTR-CM), a condition impacting approximately 400,000 to 500,000 patients worldwide. The companies are presently conducting what Monia describes as the “largest study ever conducted” in ATTR-CM, with the trial’s results anticipated in 2025, according to Dobber.
Among the smaller group of ATTRv-PN patients, Wainua faces competition from Onpattro by Alnylam, the initial medication sanctioned in 2018 for this condition. Although Alnylam shared similar aspirations for ATTR-CM as Ionis and AstraZeneca, it abandoned its pursuit of broadening its label following the FDA’s rejection last year.
In the ATTR-CM domain, Pfizer’s highly successful tafamidis, marketed as Vyndamax and Vyndaqel, gained approval in 2019, making it the pioneer in this field. Several analysts observed in September 2022 that Pfizer’s initial market presence could be challenging to overcome, describing it as firmly established, especially as Alnylam seemed ready to expand into the ATTR-CM treatment sector.
AstraZeneca and Ionis have teamed up globally to develop and market Wainua, aiming to treat ATTRv-PN in the US while pursuing approval across Europe and other global regions. This partnership has grown, granting AstraZeneca exclusive rights to distribute Wainua in Latin America and all territories beyond the US. The drug has received Orphan Drug Designation in both the US and EU for treating ATTR. Wainua is set to be accessible in the US starting from January 2024.
Brett P. Monia, Ph.D., CEO of Ionis, expressed that the FDA approval of Wainua is a significant achievement for individuals grappling with hereditary transthyretin-mediated amyloid polyneuropathy. This approval signifies the availability of a highly effective and well-tolerated treatment that can be self-administered through an auto-injector, providing a valuable resource in the fight against this debilitating disease. Monia highlighted the importance of this milestone for Ionis, as Wainua is set to be the initial product in a series of potential commercial launches for the company. Monia conveyed pride in Ionis’s collaboration with AstraZeneca in the discovery and development of Wainua, expressing gratitude to patients, caregivers, investigators, and the dedicated scientists and researchers involved in the clinical studies.
Wainua is presently undergoing assessment in the Phase III CARDIO-TTRansform trial for managing transthyretin-mediated amyloid cardiomyopathy (ATTR-CM). This is a systemic, advancing, and life-threatening condition that commonly results in gradual heart failure, frequently culminating in death within three to five years from the onset of the disease.

Downloads
Article in PDF



