Unleashing the Power of mRNA: A Revolutionary Approach to Vaccines and Therapeutics
Feb 02, 2024
Table of Contents
The progress of mRNA-based therapeutics involves several key stages, including mRNA design, synthesis, entrapment, pharmacodynamics, pharmacokinetics, in vivo and in vitro safety evaluation, manufacturing, and clinical trials. Among these stages, mRNA design and synthesis play pivotal roles in developing mRNA-based medicines. The application of mRNA for therapeutic purposes holds significant promise in addressing a diverse range of incurable diseases. Recent advancements in biotechnology and molecular medicine have empowered the generation of virtually any functional protein or peptide within the human body through the introduction of mRNA as a vaccine or therapeutic agent. This marks the emergence of a burgeoning field in precision medicine, offering considerable potential for preventing and treating challenging genetic diseases.
In recent decades, particularly in the last few years, substantial scientific progress has been witnessed in the realm of mRNA-based therapeutics. Ongoing clinical endeavors involving mRNA-based drugs are focused on areas such as infectious disease vaccines, cancer immunotherapies, therapeutic protein replacement therapies, and the treatment of genetic diseases. To harness the full potential of mRNA drugs as potent and versatile tools against diseases, it is imperative to enhance the optimization technology of mRNA structure and engineer precise nanoparticles for mRNA-based therapeutics.
Downloads
Article in PDF
Recent Articles
The landscape of clinical trials featuring therapeutic mRNA cancer vaccines is rapidly expanding, capitalizing on recent research breakthroughs that have refined mRNA delivery methods. Despite notable advancements, challenges persist in achieving optimal mRNA vaccine immunogenicity and efficacy.
The Vaccine Revolution: mRNA Vaccines Take Center Stage
In 1996, the initial exploration of mRNA-based cancer vaccines involved testing dendritic cells loaded with RNA in a laboratory setting. Subsequent advancements in technology have enhanced the structure, stability, and delivery methods of mRNA, leading to ongoing clinical trials that are actively recruiting cancer patients for mRNA-based vaccine therapies. Various administration routes for mRNA vaccines include intradermal, subcutaneous, intranasal, intranodal, intramuscular, intratumoral, and intravenous delivery.
While the conventional approach involves ex vivo engineering of autologous dendritic cells with mRNA for tumor antigen delivery, a predominant focus in most mRNA vaccine strategies is on the direct administration of mRNA using lipid nanoparticulate formulations as carriers. The potential of mRNA vaccines as promising therapeutic options for future cancer treatments is underscored, particularly when combined with additional immunotherapies. The primary objective of mRNA-based vaccination is to initiate or enhance a potent anti-tumor immune response.
The scope of clinical trials involving therapeutic mRNA cancer vaccines is swiftly growing, capitalizing on recent research breakthroughs that have enhanced mRNA delivery, streamlined administration procedures, and boosted translational efficiency. Despite notable advancements, there are still various hurdles to overcome in enhancing the immunogenicity and effectiveness of mRNA vaccines. A key breakthrough in therapeutic clinical cancer vaccines lies in the capability to pinpoint unique cancer neoantigens. Nonetheless, identifying tumor-specific mutations or unconventional sequences and predicting corresponding neoepitopes for individual HLA alleles continue to pose challenges.
Moreover, addressing the forthcoming challenges of swiftly and extensively implementing good manufacturing practices for the production of personalized mRNA vaccines requires overcoming both technological and regulatory barriers.
The majority of cancer vaccines based on mRNA primarily serve a therapeutic purpose rather than a preventative one. These vaccines necessitate repeated doses and a significant potency level to elicit a response against tumors when used in isolation. While mRNA-based vaccines as standalone treatments may prove effective for individuals diagnosed with early-stage cancer or in an adjuvant context, their success as sole therapies for advanced cancers seems improbable due to difficulties associated with the strongly immunosuppressive tumor microenvironment in such cases.
Beyond Immunity: mRNA in Therapeutics
In recent years, the groundbreaking discovery and application of mRNA technology in vaccine development has revolutionized the field of immunology. However, the potential of mRNA extends far beyond its role in immunity. Researchers are now exploring the vast therapeutic possibilities of mRNA, opening new avenues for treating a wide range of diseases and disorders.
Traditionally, mRNA was viewed as a transient intermediary in the cellular process of protein synthesis. The emergence of mRNA therapeutics marks a paradigm shift, utilizing the inherent capabilities of mRNA to produce therapeutic proteins within the body. This novel approach eliminates the need for traditional drug manufacturing and delivery systems, offering a more direct and personalized method of treatment.
Cancer Immunotherapy
One of the most promising applications of mRNA therapeutics lies in the realm of cancer treatment. Researchers are developing mRNA-based cancer vaccines that instruct the body’s immune system to recognize and target cancer cells specifically. This approach holds the potential to revolutionize cancer immunotherapy, providing a highly targeted and adaptable method for combating a variety of cancers.
Rare Genetic Disorders
mRNA therapeutics also show promise in addressing rare genetic disorders caused by mutations resulting in specific proteins’ absence or malfunction. By delivering the correct mRNA sequences into the cells, researchers aim to enable the production of functional proteins, effectively treating the underlying genetic cause of these disorders.

Infectious Diseases
Beyond vaccines, mRNA technology is being explored for the development of antiviral therapies. The ability to rapidly design and produce mRNA sequences targeting specific viral components allows for a swift response to emerging infectious diseases. This adaptability is particularly crucial in the face of evolving pathogens and emerging viral threats.
Neurological Disorders
The blood-brain barrier has long presented a challenge for drug delivery to the central nervous system. mRNA therapeutics, however, offer a potential solution. Researchers are investigating the use of mRNA to encode therapeutic proteins that can cross the blood-brain barrier, providing a new avenue for treating neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease.
While the potential of mRNA therapeutics is immense, several challenges remain to be addressed. Ensuring the stability and efficient delivery of mRNA molecules, minimizing off-target effects, and addressing potential immune responses are among the key hurdles that researchers are actively working to overcome.
Emerging mRNA-based Vaccines and Therapeutics
Significant developments have been made in mRNA therapy over the past several decades. Many monotherapies and combinations are in trial. Companies are diligently working toward the development of novel mRNA-based treatments. Key players such as BioNTech SE (BNT111, BNT116), ModernaTX (mRNA-4157, mRNA-4359), CureVac (CV8102), Sanofi (SAR441000), and others are some of the major players that are going to boost the mRNA-based vaccines and therapeutics market dynamics in the coming years.
BNT111 is an mRNA-based cancer vaccine designed to encode a specific set of four antigens associated with melanoma. Its primary goal is to stimulate a robust and targeted immune response in individuals diagnosed with advanced melanoma. The vaccine is currently undergoing evaluation in a Phase II clinical trial in combination with cemiplimab, a PD-1 inhibitor jointly developed by Regeneron and Sanofi. The study is focused on patients with unresectable Stage III or IV melanoma who have shown resistance or relapse following anti-PD1 treatment. In September 2021, the FDA granted Orphan Drug Designation to BNT111, and in November 2021, it received Fast Track Status. Early findings from the Phase I Lipo-MERIT trial indicated a promising safety profile and preliminary evidence of anti-tumor responses among patients with advanced melanoma treated with BNT111.
BioNTech and Regeneron are extending their collaborative efforts to advance the development of BNT116 along with Libtayo (cemiplimab) for the treatment of advanced non-small cell lung cancer (NSCLC). BNT116, an investigational mRNA-based cancer vaccine, is based on BioNTech’s FixVac platform. It consists of a fixed combination of tumor-linked antigens commonly found in NSCLC. The initial phase of the partnership involves initiating Phase I/II trials for BNT116 plus Libtayo as a first-line therapy for advanced NSCLC. Currently, BNT116 is undergoing evaluation in Phase I/II trial, both as a standalone treatment and in combination with cemiplimab, for first-line treatment of individuals with advanced non-small cell lung cancer whose tumors express PD-L1 in ≥ 50% of tumor cells. The study is expected to be completed by March 2027.
mRNA-4157/V940 represents an innovative investigational cancer vaccine employing mRNA technology. It is tailored to each patient by utilizing a synthetic mRNA containing coding for a maximum of 34 neoantigens. These neoantigens are determined based on the distinctive mutational pattern found in the patient’s tumor DNA sequence. Upon administration, the algorithmically generated RNA-encoded neoantigen sequences are internally translated within the body. They then undergo natural cellular processes for antigen presentation, a crucial step in adaptive immunity.
Presently, the vaccine is undergoing evaluation in a Phase II trial in conjunction with KEYTRUDA, specifically for individuals with high-risk melanoma. The company has disclosed encouraging top-line Phase II results, revealing that the combined treatment of mRNA-4157/V940 with KEYTRUDA exhibited a statistically significant and clinically meaningful enhancement in the primary outcome of recurrence-free survival compared to KEYTRUDA alone. This applies to adjuvant treatment in patients with stage III/IV melanoma following complete resection.
CV8102, a TLR7/8/RIG-1 agonist utilizing noncoding single-stranded RNA, is formulated to influence the tumor microenvironment following intratumoral administration. It aims to trigger a systemic immune response capable of managing both locally injected and remotely situated lesions. Currently undergoing investigation in a Phase I study with an open-label design, dose escalation, and expansion, CV8102 is recruiting patients with advanced melanoma, cutaneous squamous cell carcinoma, squamous cell carcinoma of the head and neck, or adenoid cystic carcinoma, specifically targeting superficially injectable tumor lesions. Initial findings from the concluded Phase I expansion study focused on individuals with PD-1 refractory melanoma, affirm the strong safety profile of CV8102 when administered alone or in conjunction with anti-PD-1 antibodies. Moreover, early indications of efficacy were noted in a subset of 30 patients treated with a combination of CV8102 and anti-PD-1 antibodies, with 40% having received prior treatment with anti-CTLA-4 antibodies.
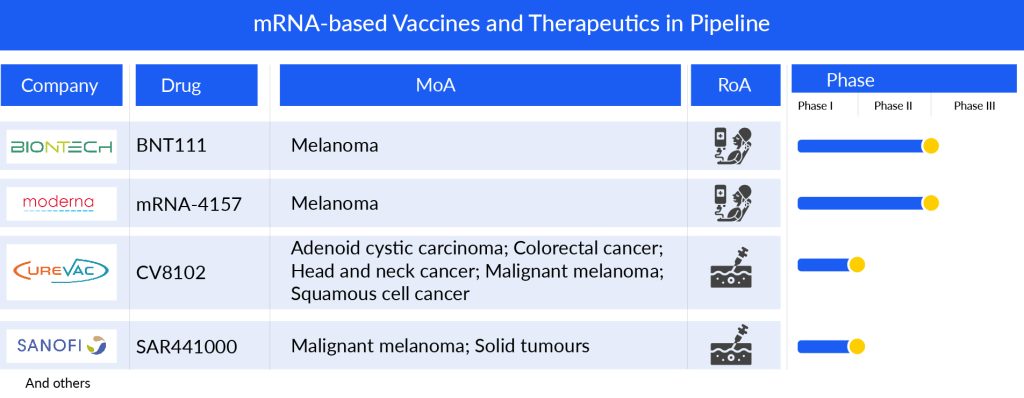
SAR441000 represents an innovative blend of four mRNA types—interleukin-12 single chain, interferon alpha-2b, granulocyte-macrophage colony-stimulating factor, and interleukin-15 sushi—formulated in saline. Developed in partnership with BioNTech, this experimental compound is currently undergoing Phase I clinical trials involving patients with advanced solid tumors. The study is expected to conclude by March 2024.
Future Horizons: mRNA in the Era of Personalized Medicine
In the rapidly evolving landscape of medical science, the advent of mRNA technology has heralded a new era in personalized medicine, offering unprecedented opportunities for targeted and individualized treatment approaches. The future horizons of medicine are being shaped by the remarkable capabilities of mRNA, a versatile molecule that plays a pivotal role in cellular processes. This revolutionary technology has transcended traditional boundaries, unlocking potential therapeutic avenues that were once deemed challenging or impossible.
One of the most groundbreaking applications of mRNA technology lies in the development of personalized vaccines. The success of mRNA vaccines in combating infectious diseases, such as the COVID-19 pandemic, has underscored the adaptability and efficacy of this approach. The ability to swiftly design and produce mRNA vaccines tailored to specific pathogens positions personalized medicine at the forefront of global health security, offering a rapid response to emerging threats.
Beyond infectious diseases, mRNA is poised to transform the landscape of cancer treatment. The precision and customization afforded by mRNA therapies enable the targeting of specific cancer antigens, minimizing collateral damage to healthy cells. Personalized mRNA cancer vaccines hold promise for inducing a robust immune response against tumor cells, offering new hope for patients in their battle against cancer.
Furthermore, the versatility of mRNA extends into the realm of genetic disorders. By harnessing the power of mRNA, scientists are exploring the potential to correct genetic mutations at the source. This opens avenues for treating a myriad of genetic diseases at their root cause, heralding a paradigm shift from symptom management to curative interventions.
However, the realization of these future horizons in mRNA-based personalized medicine requires continued research, innovation, and ethical considerations. Striking a balance between scientific advancement and ethical implications is crucial to harness the full potential of this transformative technology. As the field progresses, interdisciplinary collaboration among scientists, clinicians, ethicists, and policymakers will be essential to navigate the complexities and ensure responsible and equitable implementation of mRNA therapies.

Downloads
Article in PDF