Gene Therapies as a Game-Changer in Ophthalmology: Eyeing the Future
Mar 04, 2024
Table of Contents
Gene therapy is becoming a promising solution for retinal degenerative illnesses, particularly because the retina offers an excellent avenue for studying and treating eye-related issues. Importantly, it is the first tissue in the United States to receive approval for gene therapy in cases of inherited disorders.
Gene therapy presents a distinctive chance to manage and potentially eradicate retinal degenerative illnesses, bringing optimism to the millions affected by genetic conditions or carrying mutations that cause disease. Nevertheless, numerous obstacles must be overcome before gene therapy can be extensively accessible for treating eye diseases. Key steps to progress treatments for inherited retinal diseases and transform the realm of visual impairment include addressing the ideal timing for intervention, establishing standardized ways to measure outcomes, reducing inflammation, raising awareness, and ensuring fair access.
Downloads
Article in PDF
Recent Articles
- Toujeo to treat Type 1 Diabetes; TauRx for Alzheimer’s patients
- DelveInsight’s Gastrointestinal based Gene Therapy Reports
- VBL Therapeutics’ VB-111 (ofranergene obadenovec); BMS’s mavacamten (Camzyos); Merck’s KEYTRUDA; ...
- Actinium Announces SIERRA Trial Results; Santhera Seeks FDA Review for Vamorolone; Seres Announce...
- Metachromatic Leukodystrophy (MLD): A Rare Indication with great unmet medical need
As gene therapy finds the eye to be an optimal target, progress in this domain is expected to quicken. There’s a strong likelihood that forthcoming advancements will pave the way for potent gene therapy solutions, potentially culminating in the eradication of specific eye-related diseases.
Shedding Light on Inherited Retinal Disorders
Inherited retinal disorders (IRDs) encompass a spectrum of genetic conditions that lead to progressive vision loss and, in severe cases, blindness. These disorders, including retinitis pigmentosa and Leber congenital amaurosis, are caused by mutations in genes crucial for retinal function. Gene therapy aims to address these mutations by delivering functional copies of the defective genes into retinal cells, restoring vision and halting disease progression.
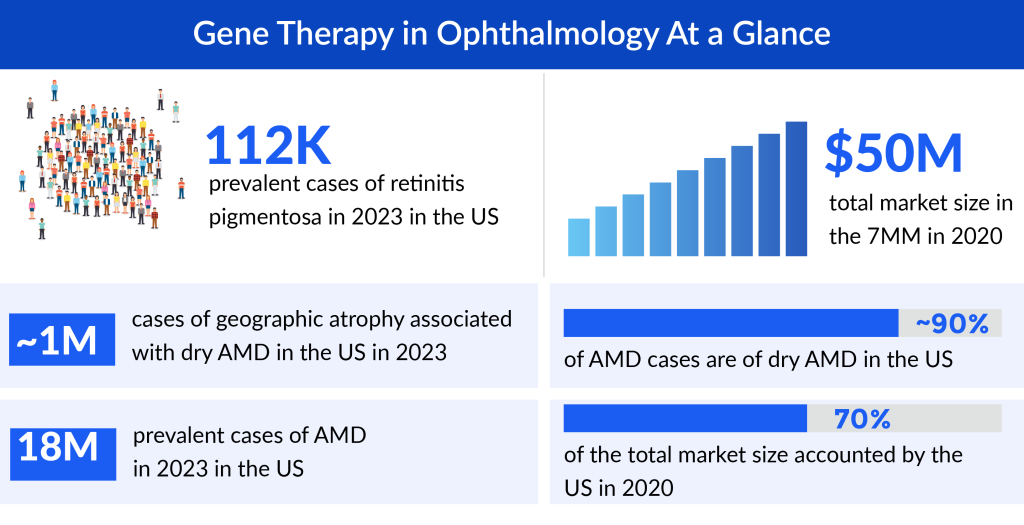
IRDs collectively affect a significant number of individuals worldwide, with varying prevalence rates among different populations. While estimates vary, it’s believed that IRDs collectively affect around 1 in 2,000 to 1 in 5,000 individuals. However, the prevalence can be higher in certain populations due to genetic factors and consanguinity.
Retinitis pigmentosa, the leading cause of visual disability and blindness in subjects less than 60 years old. According to DelveInsight’s estimates, the US accounted for around 112K prevalent cases of retinitis pigmentosa in 2023. These cases are expected to increase during the forecast period. As per the estimated, dry AMD is more prevalent than wet AMD accounting for ~90% of AMD cases in the US.
The impact of IRDs extends beyond the affected individuals to their families and communities. Vision loss, often starting in childhood or early adulthood, can profoundly affect one’s quality of life, independence, and emotional well-being. Families coping with IRDs face numerous challenges, from navigating complex healthcare systems to making lifestyle adjustments to accommodate visual impairments. The uncertainty of disease progression adds an additional layer of stress, requiring ongoing support and access to specialized care. Despite the challenges posed by IRDs, significant strides have been made in understanding these disorders and developing potential treatments.
Luxturna: A Glimmer of Hope
The approval of LUXTURNA (voretigene neparvovec), developed by Spark Therapeutics, marked a significant milestone in the field of gene therapy for ophthalmic disorders. Luxturna became the first FDA-approved gene therapy for the treatment of inherited retinal diseases, specifically targeting RPE65 mutations associated with Leber congenital amaurosis and retinitis pigmentosa.
LUXTURNA is an AAV vector-based one-time gene therapy. Patients must have viable retinal cells, as determined by the treating physician. Mutations in the RPE65 gene lead to reduced or absent levels of RPE65 isomerohydrolase activity, blocking the visual cycle and resulting in impairment of vision. Injection of LUXTURNA into the subretinal space results in the transduction of some retinal pigment epithelial cells with a cDNA encoding normal human RPE65 protein, thus providing the potential to restore the visual cycle.
Spark Therapeutics’ LUXTURNA received approval from the FDA in December 2017. The European Commission (EU) granted approval for LUXTURNA in November 2018, with authorization extending to all 28 EU member states, as well as Iceland, Liechtenstein, and Norway.
LUXTURNA stands as the pioneering FDA-approved gene therapy for a hereditary condition, marking the inception of pharmacological treatment for inherited retinal diseases. It also holds the distinction of being the first AAV vector gene therapy sanctioned in the United States. Following LUXTURNA’s approval, numerous companies ventured into gene therapy within the ophthalmology realm, aiming at various genes responsible for a spectrum of disorders like retinitis pigmentosa, LCA, LHON, achromatopsia, choroideremia, AMD (both wet and dry forms), diabetic retinopathy, DME, among others.
Promising Gene Therapies Targetting Various Eye Disorders
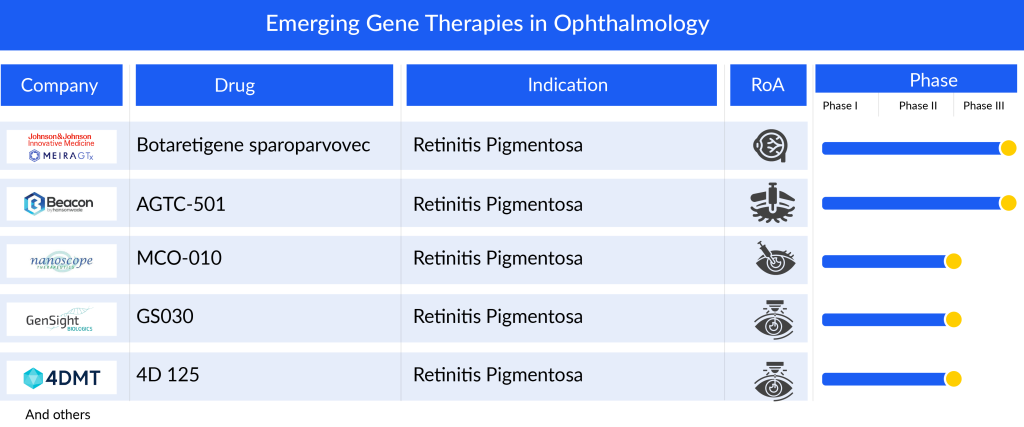
Several prominent companies are working in the gene therapy domain to improve the treatment landcape of the respective eye disorders. In the retinitis pigmentosa pipeline among the emerging candidates, Beacon’s AGTC-501 aims to restore RPGR gene function in patients with X-linked retinitis pigmentosa. In December 2023, Beacon presented unmasked three-month data from its randomized, controlled, multicenter Phase II SKYLINE trial of AGTC-501 in patients with X-linked retinitis pigmentosa at the FLORetina ICOOR 2023. The landscape for diabetic retinopathy and DME therapeutic development is currently limited, with only a few noteworthy players such as AbbVie/REGENXBIO’s ABBV-RGX-314 and 4D Molecular Therapeutics (4D-150).
Some of the drugs in the pipeline include LUMEVOQ (GenSight Biologics), Botaretigene sparoparvovec (MeiraGTx Limited), and JNJ-8120188 (Johnson & Johnson), OCU400 (ocugen), 4D-150 (4D Molecular Therapeutics), ASTSN-101 (Astena Therapeutics), and others. The detailed assessment of some of these gene therapies are given below
LUMEVOQ (GS010; lenadogene nolparvovec): GenSight Biologics
LUMEVOQ is designed to target Leber Hereditary Optic Neuropathy (LHON) by utilizing a specialized technology platform called mitochondrial targeting sequence (MTS), which originated from research at the Institut de la Vision in Paris. This technology, when combined with the specific gene related to the condition, enables the platform to address mitochondrial defects using an AAV vector.
In April 2023, GenSight Biologics decided to withdraw its application for LUMEVOQ from the European Medicines Agency. The company cited considerations from the EMA’s Committee for Advanced Therapies (CAT) regarding the medication’s benefits as the reason for the withdrawal. GenSight now plans to submit an application for marketing authorization in the UK in the second half of 2024, with hopes for a decision by the second half of 2025. Simultaneously, GenSight will recommence discussions with the FDA to establish a regulatory pathway for approval in the United States.
Botaretigene sparoparvovec (bota-vec): MeiraGTx Limited
Bota-vec, also known as Botaretigene sparoparvovec, is developed to address the primary type of X-linked retinitis pigmentosa (XLRP) which stems from mutations in the eye-specific RPGR gene variant known as RPGR open reading frame 15 (RPGR ORF15). RPGR ORF15 is crucial for the functioning of both rod and cone photoreceptors. The Phase I/II clinical study involving bota-vec in both adult and pediatric patients has been finished, and enrollment for the Phase III Lumeos clinical trial was completed in 2023. AAV-RPGR has garnered Fast Track and Orphan Drug designations by the FDA, alongside PRIME, ATMP, and Orphan Medicinal Product designations from the EMA.
JNJ-81201887: Johnson & Johnson
JNJ-81201887, previously known as AAVCAGsCD59, is a gene therapy being investigated for the treatment of individuals with geographic atrophy resulting from dry age-related macular degeneration. This therapy, JNJ-1887, is formulated to enhance the production of a soluble version of CD59 (sCD59) with the aim of safeguarding retinal cells to impede and forestall the advancement of the disease. The Phase II clinical development of JNJ-81201887 aims to assess the reduction in growth of geographic atrophy lesions in eyes treated with JNJ-81201887 in comparison to a sham control. JNJ-1887 has received Fast Track designation from the FDA and Advanced Therapy Medicinal Product (ATMP) designation from the EMA.
Overcoming Challenges: Delivery Systems and Immunogenicity
Gene therapy in ophthalmology presents an exciting frontier in medical science, offering the potential to treat debilitating eye conditions. However, this innovative approach is not without its challenges, foremost among them being the development of effective delivery systems. The eye’s unique anatomy presents hurdles in delivering therapeutic genes to the targeted cells, necessitating precise, non-invasive, and sustained methods of administration. Researchers are exploring a range of options, from viral vectors to nanoparticles, aiming to optimize delivery while minimizing side effects and ensuring patient safety.
Another significant obstacle in gene therapy for ocular diseases is the potential for immunogenicity. Introducing foreign genetic material into the eye can trigger immune responses, leading to the destruction of modified cells and diminishing treatment efficacy. Scientists are working to design gene constructs and vectors that evade immune detection, as well as developing strategies to modulate immune reactions. Overcoming these challenges requires a multidisciplinary approach, combining expertise in genetics, immunology, ophthalmology, and bioengineering to pave the way for gene therapies that offer lasting and meaningful benefits to patients with sight-threatening conditions. As research progresses, addressing these hurdles will be critical in unlocking the full potential of gene therapy for vision disorders, bringing new hope to those in need.
Looking Ahead: Expanding Horizons in Ocular Gene Therapy
Ocular gene therapy stands at the forefront of medical innovation, offering a beacon of hope for those with previously untreatable genetic eye disorders. As we look ahead into the future of this field, the horizon expands with possibilities that once seemed confined to the realm of science fiction. The promise of restoring sight to the blind, correcting genetic mutations that cause debilitating conditions like retinitis pigmentosa or Leber congenital amaurosis, is no longer a distant dream but a tangible goal within reach.
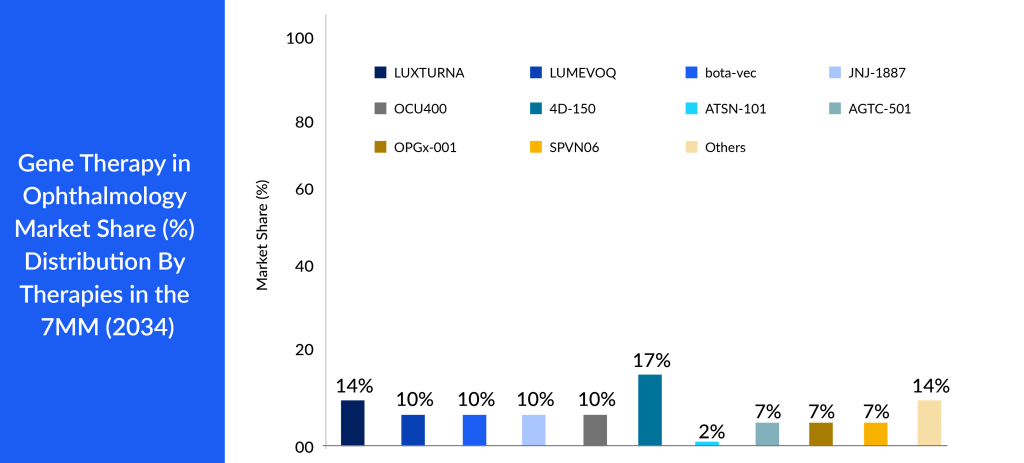
According to DelveInsight’s analysis, the market size for gene therapies in ophthalmology reached roughly USD 132 million in 2023 across the 7MM and is expected to grow with a tremendous ~58% CAGR by 2034. As per the analysis, the growth of gene therapies market in ophthalmology is expected to be mainly driven by increasing incidence of eye disease, rise in awareness and access to treatment, and robust pipeline activity for eye disease in the 7MM.
Furthermore, advancements in gene-editing technologies, such as CRISPR-Cas9, have revolutionized the precision with which we can target and modify faulty genes. This opens doors to treatments that are not only more effective but also potentially curative, addressing the root causes of diseases rather than simply managing symptoms. With ongoing research, we anticipate a widening scope of conditions that can be addressed through gene therapy, from rare inherited disorders to more common age-related eye conditions like macular degeneration.
Moreover, the expansion of horizons in ocular gene therapy also brings with it the challenge of accessibility and affordability. As these treatments become more refined and widespread, ensuring equitable distribution and affordability will be vital. Collaborations between researchers, pharmaceutical companies, regulatory bodies, and healthcare systems will play a crucial role in navigating these challenges. Nevertheless, the momentum is palpable, and the future shines bright with the potential to transform the lives of countless individuals affected by genetic eye diseases.

Downloads
Article in PDF
Recent Articles
- DelveInsight’s Gastrointestinal based Gene Therapy Reports
- FDA rejects BioMarin’s Valoctocogene Roxaparvovec; J&J inks $6.5B deal; Alzheon bags $...
- What is driving Retinitis pigmentosa market forward?
- HUTCHMED’s NDA Submission to FDA for Fruquintinib; Cytokinetics to Discontinue ALS Drug Candidate...
- Gene Therapies at J.P. Morgan 2025: Advancing Science and Shaping the Future



