Breakthroughs in Alport Syndrome Treatment: A New Era of Hope
Mar 08, 2024
Alport syndrome, an inherited disease, predominantly manifests in its X-linked form, constituting around 80% of cases. Without intervention, roughly 90% of affected males face kidney failure by age 40, whereas females typically experience a slower progression to this condition. Many Alport syndrome cases go unnoticed due to the lack of symptoms and frequent misdiagnoses.
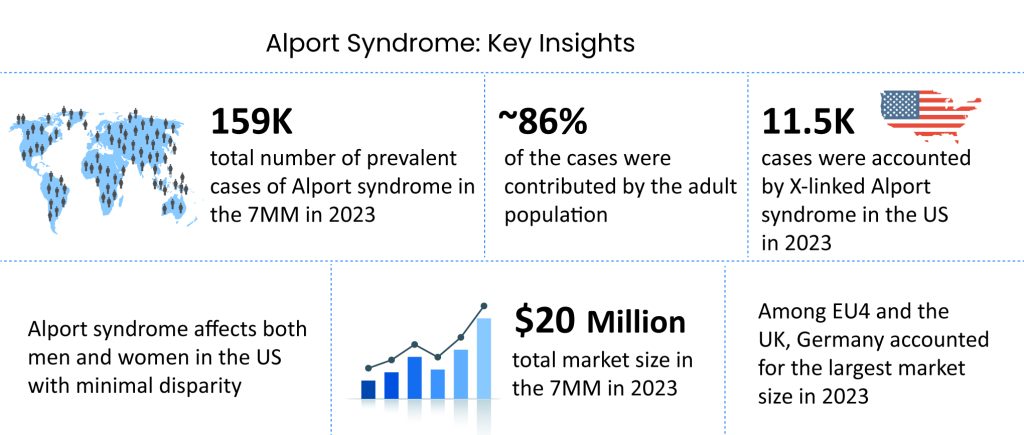
DelveInsight’s analysis reveals that the overall prevalent population of Alport syndrome in the 7MM was reported as ~159K in 2023. Within this, the population of Alport syndrome patients in the United States was specifically identified to be ~68K in the same year. As per the estimates, Alport syndrome is more prevalent in the adult population with ~86% contribution in the 7MM, while the pediatric population accounted for ~14% of cases.
Currently, ACE inhibitors and ARBs are the primary treatment approach for CKD in individuals with Alport syndrome. Nonetheless, recent years have seen a notable rise in efforts to create fresh treatment options. Several established organizations, initiated by patients, are now dedicated to advancing and supporting clinical trials for Alport syndrome. Their aim is to foster the discovery of innovative and emerging therapies for this condition.
Downloads
Article in PDF
Recent Articles
- Aslan Pharma – IQVIA collaboration; Reata’s kidney drug bardoxolone; Alzheimer’s Dise...
- Autologous cell therapy market: A new paradigm for kidney diseases
- Evolving Therapeutics in Chronic Kidney Disease (CKD) Treatment Market
- Assessment of Key Products that Got FDA Approval in Second Half (H2) of 2021
- Chronic Kidney Disease: Complex Debilitating Condition
Traditional Approaches for Alport Syndrome Treatment
Regrettably, there isn’t a specific cure for Alport syndrome. Instead, treatment aims to slow down the advancement of proteinuria and kidney disease, often involving the use of medications that target the renin-angiotensin-aldosterone system. While these Alport syndrome medications do help lower blood pressure in those with Alport syndrome, their beneficial effects are believed to come from their ability to combat fibrosis. Additional general treatments for Alport syndrome include the use of diuretics and adjustments to diet. Although these approaches might postpone the onset of kidney damage, most individuals with Alport syndrome will eventually require either dialysis or a kidney transplant.
Angiotensin-converting enzyme (ACE) inhibitors are a type of medication used to manage Alport syndrome. Historical evidence strongly indicates that starting treatment with ACE inhibitors early on can slow down the progression to end-stage renal disease in both males and females with the syndrome. However, some individuals either do not respond well to or cannot tolerate ACE inhibitors. In such cases, they may be prescribed medications known as angiotensin receptor blockers (ARBs).
Dialysis stands as a prevalent choice for those with Alport syndrome facing end-stage kidney disease (ESKD). There are two primary forms: hemodialysis, which employs a machine to filter out waste and extra fluids from the blood, and peritoneal dialysis, which utilizes the abdominal cavity lining for this filtration process. When it comes to Alport syndrome, kidney transplantation is the preferred option over dialysis, typically yielding outstanding results in the treatment of affected individuals.
Patients with ocular involvement, particularly anterior lenticonus, may opt for clear lens phacoemulsification coupled with intraocular lens placement. Cataract removal surgery is conducted when needed. Hearing aids are generally highly efficient for individuals with concurrent hearing loss. Kidney transplantation does not affect hearing loss. Adequate psychosocial support is crucial for all affected family members, as is common with any hereditary condition.
The attention towards sodium-glucose cotransporter-2 (SGLT2) inhibitors is increasing as they are seen as possible tools to slow down the advancement of chronic kidney disease. Because they are already approved for reducing blood sugar levels, there is significant interest in using these medications off-label for people with Alport syndrome. Currently, ACE inhibitors and ARBs are the primary treatments for CKD in Alport syndrome. Nevertheless, there has been a notable rise in interest in the development of new treatments in recent years.
Emerging Therapeutic Strategies For Alport Syndrome Treatment
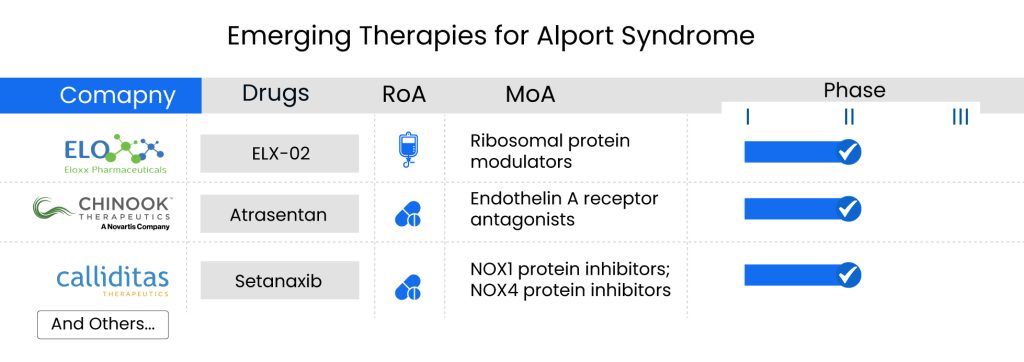
There is an urgent need for promising Alport syndrome therapies to address the significant burden. Companies including Eloxx Pharmaceuticals, Chinook Therapeutics (A Novartis Company), Bayer, Calliditas Therapeutics, Evotec, and others are investigating their potential drug candidates that can significantly change the Alport syndrome treatment market landscape during the forecast period, including ELX-02, Atrasentan, Finerenone, setanaxib, BAY3401016, and others.
The upcoming drugs, such as Eloxx’s ELX-02 is expected to be the first potential approval (gene therapy) for treating patients with Alport syndrome, followed by Novartis’ Atrasentan, which is considered as another promising therapy with strong efficacy.
ELX-02, developed by Eloxx Pharmaceuticals, is a small molecule drug aimed at reinstating the production of fully functional proteins. It targets CFTR and has shown promise in preclinical studies for treating genetic kidney diseases caused by nonsense mutations. Following encouraging Phase II trial outcomes, Eloxx plans to coordinate with the FDA on designing a pivotal trial for ELX-02 to address Alport syndrome linked to nonsense mutations, possibly aiming for Breakthrough Therapy Designation. Eloxx has recently submitted an Investigational New Drug application (IND) to the FDA for ELX-02 in treating Alport syndrome with nonsense mutations. Currently, ELX-02 is undergoing Phase II clinical trials for patients with Alport syndrome caused by nonsense mutations.
Atrasentan, developed by Chinook Therapeutics in partnership with Novartis, is a potent and highly specific blocker of the endothelin A (ETA) receptor. It shows promise in treating various chronic kidney diseases by reducing proteinuria, exerting direct anti-inflammatory actions, and mitigating fibrosis to maintain kidney function. Obtained from AbbVie in December 2019, which initially researched its use in diabetic kidney disease, atrasentan is now under investigation in the Phase II AFFINITY study for other proteinuric glomerular disorders. This comprehensive trial includes four groups: patients with IgA nephropathy with a urine protein to creatinine ratio (UPCR) of 0.5 to less than 1.0 g/g, focal segmental glomerulosclerosis (FSGS), Alport syndrome, and diabetic kidney disease. Novartis finalized its acquisition of Chinook Therapeutics in August 2023.
Finerenone, known under the brand names KERENDIA or FIRIALTA in various countries, is a selective mineralocorticoid receptor (MR) antagonist that does not belong to the steroid class. It has demonstrated the ability to counteract the harmful effects resulting from excessive MR activation. Approved for the management of chronic kidney disease associated with Type 2 diabetes in over 80 countries, finerenone shows promise in preclinical studies. Mouse experiments suggest that when combined with RAS/SGLT2 blockade, finerenone could significantly enhance renal outcomes not just in Alport syndrome but also potentially in other progressive chronic kidney diseases. The company is presently assessing the effectiveness, safety, and pharmacokinetics/pharmacodynamics (PK/PD) of an oral finerenone regimen, alongside an ACEI or ARB, for the treatment of pediatric patients aged 6 months to under 18 years with CKD and proteinuria, in Phase III clinical trial.
Introducing these promising therapies holds the potential to bring a significant ray of hope to patients grappling with this condition. The advent of such molecules is expected to alter the Alport syndrome treatment paradigm substantially.
Challenges and Future Directions in Alport Syndrome Treatment
The gene mutation that causes Alport syndrome can be silent in a person’s parents, thus making diagnosis difficult, and due to its genetic nature and the variability in symptoms, Alport syndrome is sometimes diagnosed at a later stage after significant kidney damage has occurred; more than half of the patients on current therapies reduce symptoms moderately only, significant performance remains an unmet need, with Eloxx Pharmaceuticals’ gene therapy expected to launch at premium pricing, which can create accessibility and affordability issues in some countries.
Off-label therapies, such as ACE inhibitors and ARBs, pose a significant threat to the overall Alport syndrome treatment market growth due to their potential cost burden on Alport syndrome patients and families, exacerbated by the high expense of emerging treatments. This financial strain could restrict access to essential drugs, specialized care, and even renal replacement therapies, as seen in instances such as Bardoxolone Methyl’s FDA rejection in 2022 and its trial discontinuation in Japan, alongside Sanofi’s termination of Lademirsen’s Phase II trial.
Nevertheless, with a deeper understanding of the genetic basis of Alport syndrome, personalized treatment strategies can be tailored to an individual’s specific genetic profile. ELX-02 is expected to garner the first mover advantage in the Alport syndrome treatment space apart from being the first gene therapy with reduced frequency of administration (8 weeks), amidst the usage of ACE/ARB inhibitors as the standard of care, alongside the increase in research and development paving the way for discovering novel MoA therapies such as dual NOX inhibitor (setanaxib), semaphorin-3A blocker (BAY3401016), and selective endothelin A receptor antagonist (atrasentan).
As per DelveInsight analysis, the total Alport syndrome market size in the 7MM was estimated to be ~USD 20 million in 2023, which is expected to show positive growth by 2034. Among all the therapies, ELX-02 is expected to capture the largest market size with an expected revenue of nearly USD 700 million, followed by atrasentan in the 7MM.
Furthermore, genetic advances may open the way for personalized treatment tailored to the particular genetic profiles of individuals with Alport syndrome, presenting a huge opportunity for precision medicine approaches, while patient advocacy groups such as the Alport Syndrome Foundation can play a pivotal role in shaping the landscape for Alport syndrome by directing the majority of its resources to research and research-related activities, resulting in groundbreaking knowledge and clinical trials of Alport syndrome.

Downloads
Article in PDF
Recent Articles
- Promising Data from the First Dedicated Kidney Outcomes Trial with GLP-1 Receptor Agonist, Semagl...
- Sky Medical’s Geko™ device; Fist Assist Devices obtains Breakthrough Device designation; E...
- Biogen terminates ALS Pact with Karyopharm; AbbVie’s Immunological Drug Skyrizi; NICE Backs Astel...
- Amgen Announced the Result of its CodeBreak-200 Trial; FDA Clears Bristol-Myers Squibb’s Deucrava...
- OZEMPIC’s New Approval Cements Novo’s Lead in GLP-1 Market