Potential of EGFR Inhibitors: A Promising Avenue in Cancer Treatment
Mar 29, 2024
Table of Contents
In the realm of cancer treatment, the development of targeted therapies has been nothing short of revolutionary. Among these advancements, EGFR inhibitors have emerged as a powerful weapon in the fight against certain types of cancer. EGFR, or Epidermal Growth Factor Receptor, is a protein found on the surface of cells that plays a crucial role in cell growth and division. When this signaling pathway goes awry, it can contribute to the development and progression of cancer. Enter EGFR inhibitors—medications designed to block the activity of this receptor, offering new hope and treatment possibilities for patients.
Unraveling the Science Behind EGFR Inhibitors
To truly grasp the significance of EGFR inhibitors, we must delve into the science behind them. Cancer cells often have mutations or overexpression of the EGFR gene, leading to uncontrolled cell growth. By inhibiting EGFR, these drugs disrupt the signaling pathways that drive the growth and spread of cancer cells.
Downloads
Article in PDF
Recent Articles
- Evaluating Key Advancements and Emerging Therapies in EGFR-Non Small Cell Lung Cancer Treatment M...
- HPK1 Inhibitors: A Promising Frontier in Immuno-Oncology Therapeutics
- Ofev’s expanded use; Arkin Bio-Ventures II launch; USD 125 M for COVID-19 treatment
- Vivos Therapeutics ‘s DNA Oral Appliance for the Treatment of OSA; Arthrex’s TightRope Implant fo...
- Pirtobrutinib Monotherapy: A Promising Beacon of Efficacy and Safety in Relapsed/Refractory Folli...
There are currently two types of anti-EGFR medications available. One of these is made up of small-molecule tyrosine kinase inhibitors (TKIs), while the other consists of monoclonal antibodies that target the extracellular region, preventing the receptor from dimerizing.
High levels of EGFR expression are frequently observed in various types of solid tumors such as colorectal, lung, breast, kidney, and liver cancers, often accompanied by increased levels of VEGF which are associated with worse outcomes. As a result, progress has been made in creating inhibitors that target EGFR tyrosine kinase, yet effectively incorporating these into treatment plans and selecting the most suitable patients continue to be significant hurdles.
The Impact of EGFR Inhibitors on Non-Small Cell Lung Cancer
Various generations of EGFR inhibitors are utilized to treat a range of cancers including breast, colon, lung, and pancreatic cancer. The latest versions are designed with improved access to the central nervous system, specifically for addressing brain metastases, and they also offer increased selectivity, which helps in reducing the side effects associated with inhibiting the wild-type EGFR. These advancements in treatment aim to enhance both the tolerability and effectiveness of therapies, potentially improving the quality of life for patients compared to traditional chemotherapy. It is essential to identify EGFR mutations through molecular profiling to provide personalized and high-quality therapy, especially in cases of NSCLC.
Even though EGFR-mutated lung cancer is known for its aggressive behavior, it is typically manageable, particularly due to the introduction of new treatment modalities in recent times. The utilization of EGFR-TK inhibitory mechanisms has transformed the landscape of cancer therapy. Among these are specialized treatments known as EGFR tyrosine kinase inhibitors (TKIs), such as TARCEVA (erlotinib), IRESSA (gefitinib), GILOTRIF (afatinib), TAGRISSO (osimertinib), and RYBREVANT (amivantamab).
Giotrif (afatinib) is a prescribed oral medication used as an antineoplastic treatment for locally advanced or metastatic non-small cell lung cancer. Boehringer Ingelheim obtained FDA approval for Afatinib in July 2013, marking it as the primary treatment option for patients with metastatic NSCLC who have specific epidermal growth factor receptor mutations.
TAGRISSO (osimertinib) is a third-generation EGFR tyrosine kinase inhibitor created to address EGFR-mutated NSCLC in patients, especially those with the T790M mutation, typically prescribed after resistance to previous-generation EGFR inhibitors emerges. In April 2018, the FDA approved TAGRISSO for initial treatment in patients with metastatic NSCLC whose tumors possess EGFR exon 19 deletions or exon 21 L858R mutation, as identified through an FDA-endorsed test.
These EGFR inhibitors have shown remarkable efficacy in NSCLC patients, leading to improved outcomes and quality of life. They have become a standard of care in the first-line treatment of EGFR-mutant NSCLC, often outperforming traditional chemotherapy in terms of response rates and progression-free survival.
Extending EGFR Inhibitors Benefits to Colorectal Cancer
Initially established as a cornerstone of treatment for EGFR-mutant lung cancers, these inhibitors have demonstrated promising efficacy in a subset of colorectal cancer patients with specific mutations. By targeting the EGFR pathway, these inhibitors disrupt the signaling cascades that fuel tumor growth, presenting a tailored approach to combatting this disease. Through ongoing research and clinical trials, scientists are unraveling the complex molecular profiles of colorectal tumors, identifying those most likely to respond to EGFR inhibitors and expanding the therapeutic options for patients.
The translation of EGFR inhibitor success from lung cancer to colorectal cancer underscores the evolving landscape of targeted therapies. One of the key mutations studied in colorectal cancer is the KRAS mutation, which has historically limited the effectiveness of EGFR inhibitors. However, newer generations of these inhibitors, coupled with sophisticated biomarker testing, have offered renewed hope. Identifying patients with wild-type KRAS status has become pivotal, as they are more likely to benefit from EGFR inhibition. This nuanced understanding allows oncologists to personalize treatment plans, optimizing outcomes and potentially extending survival rates for colorectal cancer patients.
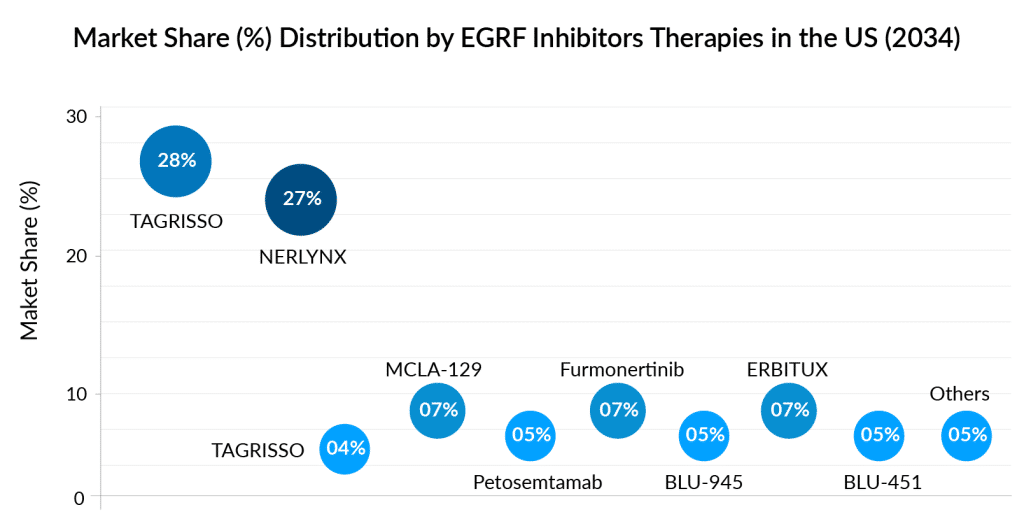
Moreover, the extension of EGFR inhibitors to colorectal cancer exemplifies the era of combination therapies and precision oncology. Combinations of EGFR inhibitors with other targeted agents or traditional chemotherapy have shown synergistic effects, enhancing response rates and delaying disease progression. This multifaceted approach not only addresses the heterogeneity of colorectal cancer but also highlights the importance of tailored treatment strategies. As research continues to uncover novel biomarkers and therapeutic combinations, the vision of extending the benefits of EGFR inhibitors to a broader spectrum of colorectal cancer patients moves closer to reality, promising new avenues in the quest for improved outcomes and quality of life.
Promising EGFR Inhibitors on the Horizons
Some of the drugs in the pipeline include Petosemtamab (Merus), MCLA-129 (Merus), and Zipalertinib (Cullinan Oncology/Taiho Pharma), Furmonertinib (Arrivent Biopharma), BLU-945 (Blueprint Medicines Corporation), and others.
Petosemtamab (Merus), a bispecific biclonics low-fucose human full-length IgG1 antibody, is designed to target both the EGFR and the leucine-rich repeat containing G-protein-coupled receptor 5 (LGR5). It is currently under development for potential use in treating HNSCC. The ongoing Phase I/II clinical trial of petosemtamab is now in its dose expansion phase, conducted as an open-label, multicenter study. The trial is actively enrolling patients with HNSCC, exploring the efficacy of petosemtamab alone in those previously treated for HNSCC, as well as in combination with KEYTRUDA (pembrolizumab) for untreated cases of HNSCC.
The company’s presentation outlines plans for a Phase III monotherapy trial set to begin in mid-2024. Updates on the clinical progress of second-line treatment with petosemtamab are slated for 2024, alongside expectations for updates on combination therapy in the first half of that same year.
MCLA-129 another Merus lead asset, is an ADCC-enhanced human IgG1 biclonics, is designed to target EGFR and c-MET. Merus has granted exclusive development and potential commercialization rights of MCLA-129 in China to Betta Pharmaceuticals, while retaining complete rights for all other regions. The drug is currently undergoing Phase I/II trials for the treatment of advanced NSCLC and various solid tumors. Patient enrollment for dose expansion cohorts is ongoing, examining the effectiveness of MCLA-129 as a monotherapy in MET ex14 NSCLC, in combination with TAGRISSO for treatment-naïve EGFR mutant (m) NSCLC, and for EGFRm NSCLC that has progressed after TAGRISSO. Additionally, trials are assessing MCLA-129 combined with chemotherapy for 2L+ EGFRm NSCLC. The company’s presentation indicates plans to begin combination therapy with chemotherapy in the first quarter of 2024.
Zipalertinib (CLN-081/TAS6417) by Cullinan Oncology/Taiho Pharma is a new type of EGFR inhibitor that can be taken by mouth and works in a way that cannot be reversed. In laboratory studies, it has shown a strong ability to target and affect cells that have EGFRex20ins mutations, while being gentler on cells with the regular EGFR, aiming to prevent the negative effects that can come from inhibiting the normal EGFR function. By 2024, the crucial Phase IIb trial focusing on treating NSCLC after the second line and beyond was completely filled with participants. At the same time, enrollment is actively happening for the Phase III trial which aims to treat NSCLC with exon 20 insertion mutations right from the start of treatment.
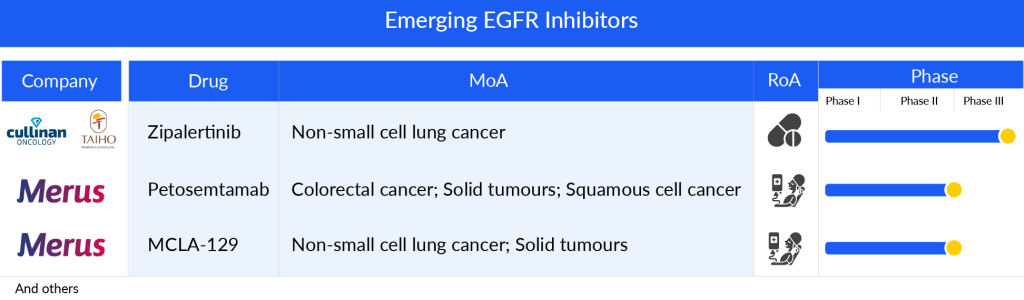
The anticipated launch of these emerging therapies are poised to transform the EGRF inhibitors market landscape in the coming years. As these cutting-edge therapies continue to mature and gain regulatory approval, they are expected to reshape the EGRF inhibitors market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
Challenges and Future Directions of EGFR Inhibitors in Cancer Treatment
EGFR inhibitors have revolutionized cancer treatment, particularly for cancers like non-small cell lung cancer. However, alongside their success, significant challenges loom in their effective and sustainable use. One of the primary hurdles is the development of resistance. Despite initial responses, tumors often adapt through various mechanisms, rendering these inhibitors ineffective over time. This necessitates a deeper understanding of the molecular pathways involved in resistance and the development of strategies to overcome or prevent it. Combination therapies, targeting multiple pathways simultaneously, offer promise in this regard, but their optimal design and implementation require careful exploration.
Looking ahead, the future of EGFR inhibitors in cancer treatment hinges on refining patient selection criteria. Not all patients benefit equally from these drugs, and identifying biomarkers predictive of response is crucial. Precision medicine approaches, such as genetic profiling and liquid biopsies, hold potential for tailoring treatment plans to individual patients, maximizing efficacy while minimizing adverse effects. Moreover, the development of next-generation EGFR inhibitors with improved specificity and reduced toxicity profiles represents a promising avenue. These advancements aim to enhance treatment outcomes and expand the reach of EGFR inhibitors to a broader spectrum of cancer types.
In the landscape of cancer therapy, the challenge of managing side effects remains pertinent with EGFR inhibitors. Skin rash, diarrhea, and other adverse events can significantly impact patient quality of life and treatment adherence. Future directions include the development of supportive care strategies to mitigate these effects, allowing patients to benefit from the full potential of EGFR inhibitors without compromising their well-being. Additionally, ongoing research delves into the role of the tumor microenvironment in modulating drug responses, highlighting the importance of holistic approaches in cancer treatment. By addressing these challenges head-on and embracing innovative directions, EGFR inhibitors continue to hold promise as invaluable tools in the oncologist’s arsenal, offering hope to patients battling various forms of cancer.

Downloads
Article in PDF
Recent Articles
- FDA Approves Lexicon’s INPEFA (Sotagliflozin); PTC Therapeutics’s Vatiquinone MOVE-FA Registratio...
- Golcadomide and Rituximab Combo Unveils Promising Efficacy in Heavily Pretreated Relapsed/Refract...
- Unveiling the Potential of TROP-2 Inhibitors: A New Frontier in Cancer Treatment
- AstraZeneca’s Imfinzi for Biliary Tract Cancer; FDA Clears Boehringer’s Spesolimab; Novo Nordisk ...
- Colorectal Cancer Market Analysis and Market Forecast



