Cervical Cancer Treatment: Navigating the Changing Landscape
Nov 08, 2024
Table of Contents
Over the past two decades, the majority of countries in the 7MM have experienced a decrease in cervical cancer cases. In the United States, incidence rates declined from the mid-1970s to the mid-2000s, partly attributed to increased screening efforts.
Cervical cancer is most often diagnosed between the ages of 35 and 44. The average age of diagnosis in the US is 50. Over 20% of cervical cancers are diagnosed after age 65. It is rare for people younger than 20 to develop cervical cancer.
Downloads
Article in PDF
Recent Articles
- C4X Discovery and AstraZeneca Signs Deal; FDA Rejects Spectrum’s Poziotinib; Orphan Drug Designat...
- Insights into the Evolving Landscape of Antibody-Drug Conjugate (ADC) & the Key Companies in...
- Widespread Usage of HPV Vaccine Reduces Cervical Cancers and Precancers
- Navigating the Healthcare Horizon: Odyssey of Mergers, Funding, and Acquisitions in 2024
- Boehringer Ingelheim’s Diabetic Macular Ischemia Study; Novartis’ Mariana Oncology Acquisition; A...
As per DelveInsight analysis, the total incident cases of cervical cancer in the 7MM comprised 40K in 2023 and are projected to decrease by 2034. The US contributed to the largest incident population of cervical cancer, accounting for nearly 34% of the 7MM in 2023.
The projected decrease in total incident cases of cervical cancer in the 7MM by 2034 is attributed to the widespread implementation of HPV vaccination programs and improved screening techniques. These preventative measures significantly reduce the incidence of HPV infections, which are the primary cause of cervical cancer.

TIVDAK Approval for Cervical Cancer: A Significant Milestone
On April 30, 2024, Genmab A/S and Pfizer Inc. announced that the FDA had granted full approval to their supplemental Biologics License Application (sBLA) for TIVDAK® (tisotumab vedotin-tftv). This approval is for the treatment of patients with recurrent or metastatic cervical cancer who have experienced disease progression following chemotherapy. This decision upgrades the first accelerated approval granted to TIVDAK in September 2021 to full approval. This represents a major breakthrough in cervical cancer treatment, showcasing the promising role of antibody-drug conjugates (ADCs) in oncology.
TIVDAK is an ADC that targets tissue factor (TF). It merges Genmab’s monoclonal antibody, tisotumab, which targets TF, with Seagen’s ADC technology. This combination aims to recognize TF antigens in cancer cells and deliver the cytotoxic agent MMAE directly into these cells.
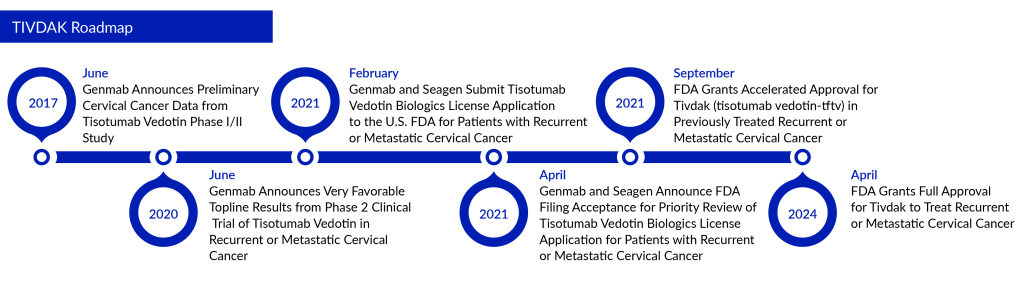
The approval is grounded on the findings from the global, randomized Phase III innovaTV 301 clinical trial (NCT04697628). In this study, TIVDAK achieved its primary goal of overall survival (OS) in patients with previously treated recurrent or metastatic cervical cancer, outperforming chemotherapy. The trial also met secondary goals, including progression-free survival (PFS) and a confirmed objective response rate (ORR). Initial results from the innovaTV 301 study were presented in October 2023 at the Presidential Symposium during the European Society of Medical Oncology (ESMO) Congress.
Other Drugs Ruling The Cervical Cancer Treatment Space
In recent years, the standard first-line treatment for persistent, recurrent, or metastatic cervical cancer has advanced. Traditionally, chemotherapy doublets, usually platinum-based, were the primary option. However, the preferred regimen now includes enhancing cisplatin-paclitaxel with bevacizumab, a VEGF monoclonal antibody. In 2014, the FDA approved Genentech/Roche’s AVASTIN (bevacizumab) for use with chemotherapy in women with advanced cervical cancer, a significant milestone as it was the first drug approved for late-stage cervical cancer since topotecan with cisplatin in 2006.
Over the past decade, biosimilars have transformed healthcare, promoting a more sustainable system. In September 2017, the FDA approved Amgen’s MVASI, the first biosimilar for cancer therapy in the US, as a counterpart to Roche’s AVASTIN, which was patent-protected until 2019.
In addition, the introduction of immunotherapies such as KEYTRUDA and LIBTAYO, enhancing early diagnosis and treatment, significantly enhanced survival rates in cervical cancer. KEYTRUDA (pembrolizumab) is a humanized monoclonal antibody that attaches to the programmed cell death-1 (PD-1) receptor, preventing its interaction with PD-L1 and PD-L2. This action lifts the PD-1 pathway-mediated suppression of the immune system, enhancing the antitumor immune response. In January 2024, the FDA approved the use of KEYTRUDA in combination therapy for patients with FIGO 2014 Stage III–IVA cervical cancer.
LIBTAYO (cemiplimab) is a completely human monoclonal antibody that targets the PD-1 receptor on T cells, created using Regeneron’s proprietary VelocImmune technology. It was developed collaboratively by Sanofi and Regeneron under a global partnership agreement. In January 2024, Medison Pharma announced a deal with Regeneron Pharmaceuticals to market LIBTAYO in selected European and other international markets.
Innovations such as targeted therapies and immunotherapies are revolutionizing cervical cancer treatment, with drugs like tisotumab vedotin representing breakthroughs. Ongoing research continues to drive advancements in therapies and diagnostics, with a focus on precision medicine.
Potential Cervical Cancer Therapies on the Horizon
Some of the drugs in the cervical cancer pipeline include TECENTRIQ (Roche), Volrustomig (AstraZeneca), PRGN-2009 (Precigen), TG4001 (Transgene), ENHERTU (Daiichi Sankyo/AstraZeneca), ISA101b (ISA Pharmaceuticals/Regeneron), and others.
TECENTRIQ: (Roche)
TECENTRIQ (atezolizumab), a prescription medicine, has shown promising results in combination with bevacizumab (AVASTIN) and chemotherapy for treating metastatic, persistent, or recurrent cervical cancer. In a phase III trial, this combination significantly improved overall survival (OS) and progression-free survival (PFS), with median OS reaching 32.1 months compared to 22.8 months with standard therapy, and median PFS extending to 13.7 months versus 10.4 months. This suggests Tecentriq could be a new frontline option; however, the combination also led to high rates of serious adverse events (SAEs) in 79% of patients, underscoring the importance of careful patient selection and management to balance efficacy and safety.
Volrustomig: AstraZeneca
Volrustomig is a bispecific monoclonal antibody administered intravenously, targeting both programmed cell death protein 1 (PD-1) and cytotoxic T lymphocyte-associated protein 4 (CTLA4). The company is currently conducting a Phase III study (NCT06079671), named eVOLVE-Cervical/eVOLVE-Cervical, to evaluate the efficacy and safety of volrustomig in women with high-risk locally advanced cervical cancer (FIGO 2018 stage IIIC to IVA with lymph node involvement). The company expects to file for approval no earlier than 2025.
PRGN-2009: Precigen
PRGN-2009, a new type of gorilla adenovirus that cannot replicate, is designed to target cancers associated with HPV. It can be given multiple times, which boosts T cell activity without raising levels of neutralizing antibodies. In May 2023, the FDA approved the Investigational New Drug (IND) application for a Phase II study of PRGN-2009. The company has now launched this Phase II trial (NCT06157151) to assess how effective and safe PRGN-2009 is when used alongside pembrolizumab, compared to using pembrolizumab alone, in patients with cervical cancer that has recurred or spread despite previous treatment with pembrolizumab.
TG4001: Transgene
TG4001 is a promising therapeutic vaccine still in the investigative stages. It’s crafted from a non-spreading, greatly weakened form of the Vaccinia virus (MVA). This vaccine is tailored to carry HPV16 antigens (E6 & E7) along with an adjuvant called IL-2. Building upon encouraging results from Phase Ib/II trials, Transgene is now progressing to a randomized Phase II trial of TG4001 combined with avelumab. Merck KGaA, Darmstadt, Germany, is contributing avelumab for this study. Initial findings from the Phase II segment were unveiled at the ASCO 2023 annual meeting, with the final outcomes slated for release in 2024. Looking ahead, Transgene is formulating plans for a potentially pivotal trial to further validate the efficacy of this innovative cancer vaccine.
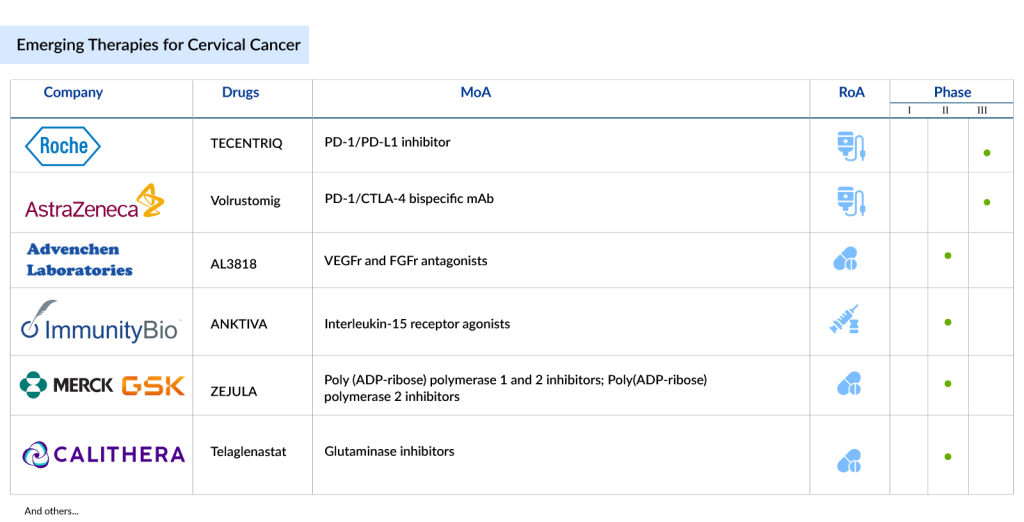
Other emerging therapies for cervical cancer treatment include Lifileucel + pembrolizumab (Iovance Biotherapeutics), VB10.16 + TECENTRIQ (Nykode Therapeutics/Roche), ISA10z1b + LIBTAYO (ISA Pharmaceuticals/Regeneron), ENHERTU (Daiichi Sankyo/AstraZeneca), and others.
The anticipated launch of these emerging therapies is poised to transform the cervical cancer treatment market landscape in the coming years. As these cutting-edge therapies continue to mature and gain regulatory approval, they are expected to reshape the cervical cancer treatment market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
The Future Cervical Cancer Treatment Market Looks Promising
Research into combining anti-PD-(L)1 agents with other checkpoint inhibitors, VEGF inhibitors, and HPV vaccines highlights the opportunity to target multiple pathways simultaneously, potentially improving treatment outcomes for cervical cancer.
New therapies for r/mCC should offer substantial clinical advantages while avoiding excessive healthcare resource utilization. Further investigation is crucial to grasp how treatment approaches for r/mCC correlate with subsequent clinical results.
A robust pipeline with a novel mechanism of action and increasing incidence are major market drivers of the cervical cancer market. Additionally, the cervical cancer pipeline is also expected to change the current dynamics of the cervical cancer treatment market, which presently comprises biologics and molecules with new mechanisms of action.
Key players like AstraZeneca and Roche, Precigen, and Nykode Therapeutics are driving progress in the cervical cancer treatment landscape. As per DelveInsight, the total market size of cervical cancer in the 7MM was approximately USD 930 million in 2023 and is projected to increase by 2034.
TIVDAK, developed by Seagen and Genmab, currently stands as the only approved antibody-drug conjugate (ADC) in cervical cancer treatment. Although it comes with a boxed warning for potential ocular side effects, TIVDAK shows promise for expansion into earlier treatment lines, particularly for patients whose tumors express the PD-L1 biomarker—an approach similar to that of Merck’s Keytruda. This shift could mark a significant advancement in cervical cancer drugs, offering targeted options that better address specific patient needs.
Looking ahead, the cervical cancer treatment landscape is set for major changes as new therapies emerge. Notably, Lifileucel, a one-time, personalized T-cell therapy, is expected to receive approval by 2025 in the US and could capture a substantial market share by 2034. With this and other targeted therapies on the horizon, the outlook for cervical cancer patients appears increasingly hopeful. The next decade is likely to bring cervical cancer drugs that use innovative, biomarker-driven approaches, enhancing outcomes both as standalone treatments and in combination with existing therapies.

Downloads
Article in PDF
Recent Articles
- The Next Chapter in NSCLC Treatment Space: Recent Discoveries and Innovations
- Widespread Usage of HPV Vaccine Reduces Cervical Cancers and Precancers
- Sanofi’s Rare Disease Drug Xenpozyme’ Approval; FDA Approves Novartis’ Pluvicto; AstraZenec...
- Bayer’s AskBio Initiates Phase II GenePHIT Trial; FDA Approves Merck’s KEYTRUDA Plus Chemoradioth...
- Boehringer Ingelheim’s Diabetic Macular Ischemia Study; Novartis’ Mariana Oncology Acquisition; A...