Oncolytic Virus Cancer Therapy: Transforming Cancer Treatment
Jul 05, 2024
Table of Contents
Oncolytic viruses (OVs) represent a new category of cancer treatments that provide several advantages: they selectively replicate within tumor cells, can deliver various eukaryotic transgene payloads, induce immunogenic cell death, enhance antitumor immunity, and generally have a safe profile that doesn’t significantly overlap with other cancer therapies.
Oncolytic viruses emerge as a way of bypassing the immune evasion mechanisms of the tumor, aiming to improve the clinical condition of patients through the stimulation of the host immune system or direct lysis of abnormal cells.
Downloads
Article in PDF
Recent Articles
- 15 Prominent Indications for Oncolytic Virus Therapy
- 8 Late and Mid Stages Adenovirus-associated Oncolytic Virus Therapies To Watch Out
- 7 HSV-associated Oncolytic Virus Therapies in Late and Mid-Stage
- Is Gene Therapy the Next Cancer Treatment Revolution?
- Oncolytic Viruses: Can Be The Next Frontier in Cancer Immunotherapy?
Oncolytic viruses are either RNA or DNA viruses. RNA viruses such as reoviruses, paramyxoviruses, and picornaviruses, which encode only a few genes, often undergo rapid proliferation and lysis of tumor cells. On the other hand, oncolytic DNA viruses such as herpes viruses, adenoviruses, or poxviruses allow for the insertion of multiple foreign genes but are slower in replication and amplification.
A significant challenge with oncolytic viruses remains the necessity of direct tumor injection, which is relatively straightforward for skin cancers like melanoma but presents greater difficulty for deeper-seated tumors. Thus, there is a need for innovation to develop oncolytic viruses that are more logistically feasible for broader patient administration, thereby expanding their therapeutic potential.
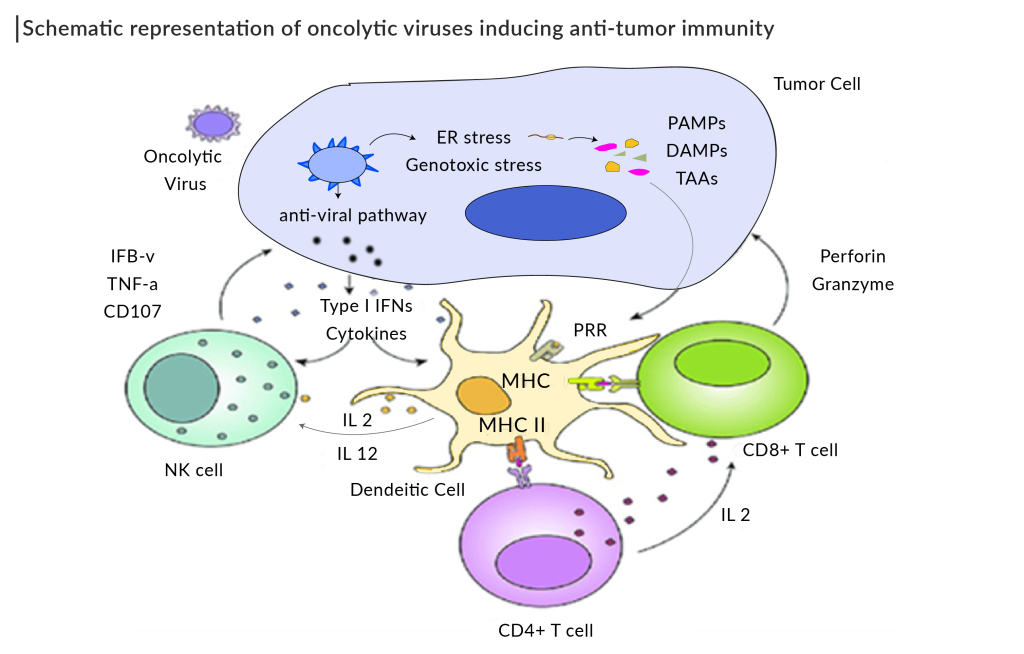
Types of Oncolytic Viruses
There are several types of oncolytic viruses currently being explored for cancer treatment. Each type of oncolytic virus offers unique mechanisms of action and therapeutic potential, making them a promising avenue for innovative cancer treatments. Some of the most common types of oncolytic viruses are:
Adenovirus
Adenoviruses, one of the most studied, are modified to replicate preferentially in cancer cells with dysfunctional p53 pathways. Adenovirus oncolytic viruses represent a promising avenue in cancer therapy, leveraging the natural virus-host interaction to target and kill cancer cells selectively. These genetically engineered viruses are designed to replicate within and lyse malignant cells, sparing normal tissues. Adenoviruses, known for their ability to infect a wide range of cell types, are modified to enhance tumor selectivity, often by inserting tumor-specific promoters or deleting viral genes necessary for replication in non-cancerous cells. This specificity minimizes collateral damage and enhances safety. Additionally, oncolytic adenoviruses can stimulate the immune system, creating an anti-tumor response that further aids in eradicating cancer cells. With ongoing research and clinical trials, adenovirus oncolytic virus cancer therapy holds substantial potential for advancing cancer treatment, offering a targeted, multifaceted approach to combat various malignancies.
Herpes Simplex Virus (HSV)
Herpes simplex virus is traditionally known for causing cold sores and genital herpes, but it has also been engineered as a type of oncolytic virus. This modified version of HSV, often referred to as oncolytic HSV, is genetically altered to enhance its ability to target tumor cells while sparing normal, healthy cells. The virus works by infecting cancer cells, replicating within them, and eventually causing cell lysis, which releases new viral particles to infect neighboring cancer cells. Additionally, the oncolytic HSV can stimulate the body’s immune system to recognize and attack the tumor, providing a two-fold approach to cancer treatment. Clinical trials have shown promise in using oncolytic HSV for treating various types of cancers, highlighting its potential as a powerful tool in the fight against cancer.
H-1 protoparvovirus (H-1PV)
H-1 protoparvovirus (H-1PV) is a type of oncolytic virus that belongs to the Parvoviridae family. Known for its natural ability to selectively infect and kill cancer cells while sparing normal tissues, H-1PV has garnered significant interest in cancer research and therapy. This small, non-enveloped DNA virus exploits the altered metabolic state of tumor cells to replicate and induce cell death, often through mechanisms such as apoptosis or autophagy. In addition to its direct oncolytic effects, H-1PV can also stimulate an anti-tumor immune response, enhancing its therapeutic potential. Preclinical and clinical studies have shown promising results in various types of cancer, positioning H-1PV as a potential candidate for future cancer treatments.
Reovirus
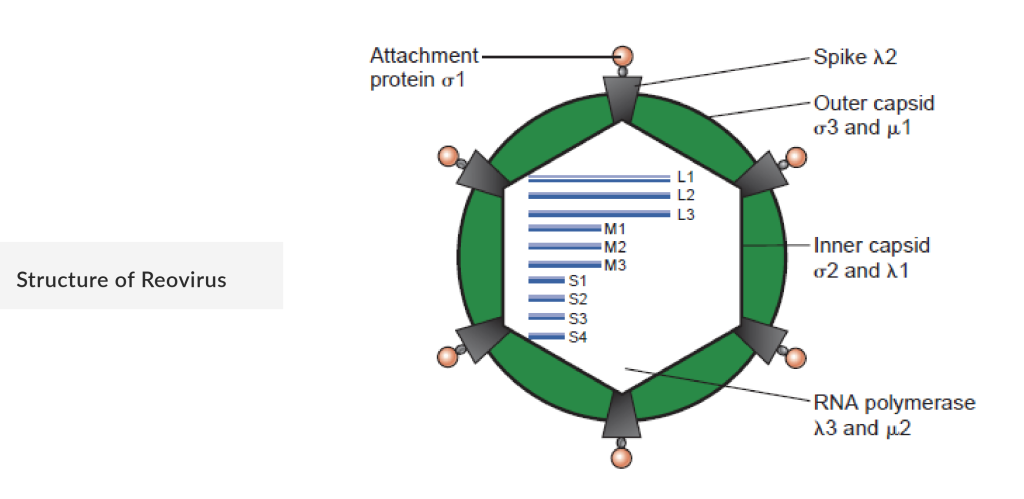
Reovirus, a naturally occurring virus, is gaining attention as a promising oncolytic virus for cancer therapy. Reovirus stands out due to its unique mechanism. It preferentially targets cells with an activated Ras signaling pathway, a common mutation in various cancers. Once inside the cancer cells, reovirus replicates, causing cell lysis and the release of new viral particles that can infect neighboring tumor cells. Additionally, the infection stimulates the body’s immune response against the tumor, enhancing the overall anti-cancer effect.
Vaccinia Virus
Vaccinia virus, a member of the poxvirus family, has garnered attention as an oncolytic virus due to its unique properties. Originally used as a vaccine against smallpox, this virus has been genetically modified for therapeutic purposes. In oncolytic virotherapy, the Vaccinia virus selectively targets cancer cells, exploiting their altered biology to replicate and induce cell death, while sparing healthy tissues. This targeted oncolytic activity is enhanced by the virus’s ability to stimulate anti-tumor immune responses, potentially amplifying its effectiveness. Ongoing research aims to optimize its therapeutic potential, offering new hope to patients suffering from various types of cancer.
Newcastle Disease Virus
Originally identified in poultry, Newcastle Disease Virus (NDV) has shown potential as a therapeutic agent in cancer treatment due to its natural preference for replicating in rapidly dividing cells, which is characteristic of many tumors. Researchers are exploring NDV’s ability to induce tumor cell apoptosis and stimulate immune responses against cancer cells, offering a dual mechanism for combating malignancies. Oncolytic virus cancer therapy clinical trials are ongoing to harness NDV’s specificity and therapeutic potential in various types of cancers, with encouraging results in enhancing patient outcomes through innovative virotherapy approaches.
Measles Virus
The measles virus is a member of the Paramyxoviridae family. This virus, known for causing the highly contagious measles disease in humans, exhibits promising therapeutic potential in cancer treatment. Its ability to target and destroy tumor cells is attributed to several factors, including its preference for cells expressing specific surface receptors that are often overexpressed on cancer cells. This targeted approach minimizes damage to healthy tissues, making the measles virus a compelling candidate for oncolytic virotherapy.
Vesicular Stomatitis Virus
Vesicular stomatitis virus is a promising oncolytic virus currently under investigation for its therapeutic potential in cancer treatment. This virus, primarily known for causing mild flu-like symptoms in livestock, has been engineered to selectively target and destroy cancer cells while leaving healthy cells unharmed. VSV’s ability to replicate specifically within tumor cells and induce their lysis makes it a valuable candidate for oncolytic virotherapy. Research is actively exploring its mechanisms of action, safety profile, and efficacy in various cancer types, holding promise for the development of innovative cancer treatments in the future.
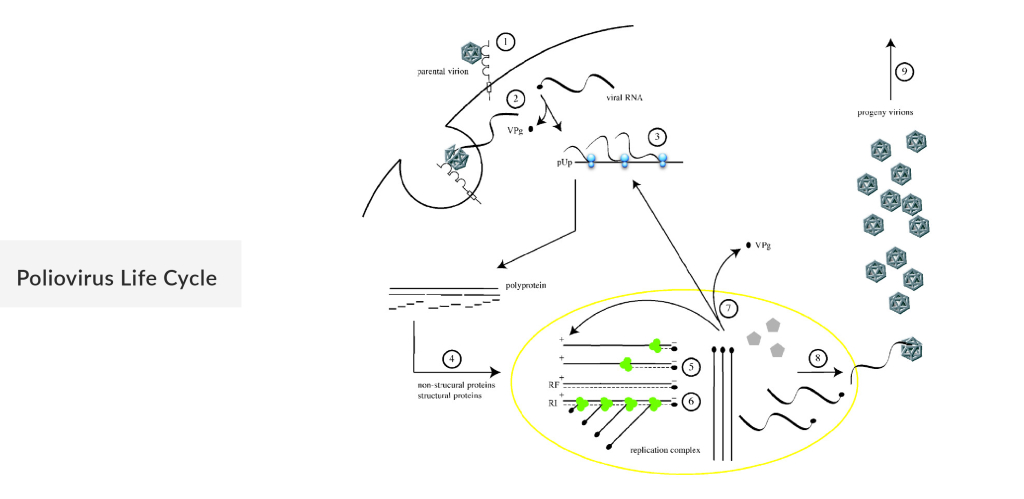
Poliovirus
Poliovirus, a virus traditionally known for causing poliomyelitis, has recently garnered attention in the field of oncology as a promising oncolytic virus. Poliovirus, genetically engineered to target malignant cells, exploits the altered molecular pathways in cancer cells to replicate and induce cell death. This process not only reduces tumor burden but also stimulates an anti-tumor immune response, enhancing the body’s ability to fight the disease. Clinical trials have shown encouraging results, particularly in treating glioblastoma, a highly aggressive brain tumor. By reprogramming poliovirus for therapeutic use, researchers are opening new avenues for cancer treatment, offering hope for improved outcomes in patients with otherwise refractory malignancies.
Picornaviruses
Picornaviruses, a group of small, non-enveloped RNA viruses, are gaining attention as a type of oncolytic virus with significant therapeutic potential. Picornaviruses, such as Coxsackievirus and Poliovirus, leverage their natural cytopathic effects to induce tumor cell lysis and stimulate anti-tumor immune responses. Their ability to replicate rapidly and spread within the tumor microenvironment makes them potent agents in oncolytic virotherapy. Additionally, their non-enveloped structure confers stability, allowing for effective delivery and infection of cancer cells.
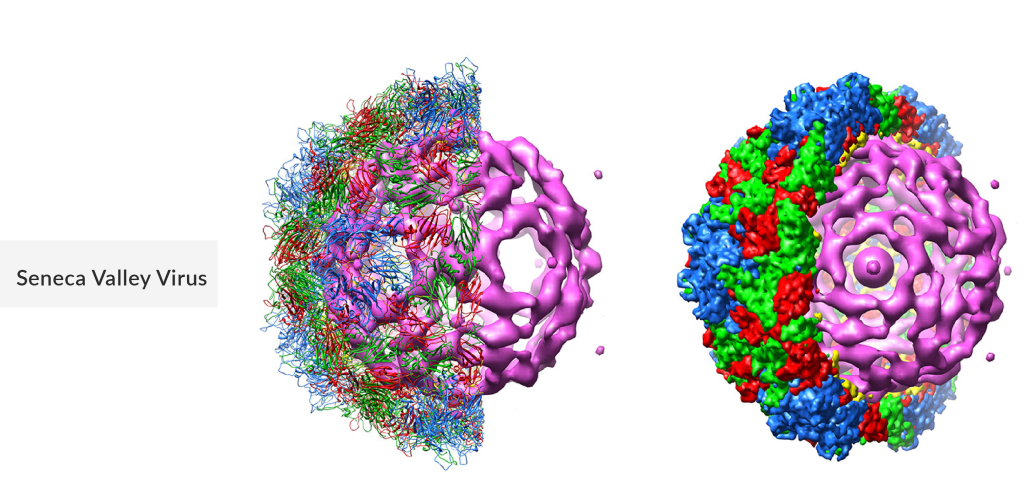
Seneca Valley Virus
Seneca Valley virus, classified as a type of oncolytic virus, exhibits a unique ability to selectively target and destroy cancer cells while leaving healthy cells largely unharmed. Initially identified in pigs, SVV has garnered attention for its potential in oncolytic therapy due to its natural tropism for neuroendocrine tumors, including small-cell lung cancer and neuroblastoma. Researchers are exploring SVV’s mechanism of action, which involves replication within cancer cells, leading to their lysis and subsequent immune system activation against the tumor. Oncolytic virus cancer therapy clinical trials are underway to assess SVV’s safety, efficacy, and broader applications in combating various types of cancers.
Oncolytic Viruses Therapy Design
The design of oncolytic viruses involves several critical components. The base virus serves as the foundation, chosen for its ability to preferentially replicate in cancer cells; common choices include adenovirus, herpes simplex virus, and vaccinia virus. Incorporation of a transgene, which encodes for therapeutic proteins such as immune modulators or apoptosis-inducing factors, enhances the anti-tumor efficacy. The transgene expression is tightly regulated by a promoter, a DNA sequence that ensures the gene is expressed predominantly within the tumor microenvironment, thereby minimizing systemic toxicity. The promoter’s specificity is vital, often derived from tumor-specific or inducible elements to maximize the therapeutic index.
For example, the construct of IMLYGIC involves deletions in both copies of the viral ICP34.5 gene, which is involved in neurovirulence and attenuation of the virus. Additionally, it incorporates the granulocyte-macrophage colony-stimulating factor (GM-CSF) gene. This gene serves to enhance the anti-tumor immune response by recruiting and activating dendritic cells, macrophages, and T cells at the tumor site.
The intricate interplay between the base virus, transgene, and promoter culminates in a sophisticated therapeutic modality designed to target and eradicate malignancies with precision.
Application of Oncolytic Viruses in Cancer Therapy
The application of oncolytic viruses spans a range of cancer types, including glioblastoma, melanoma, pancreatic cancer, and ovarian cancer. Oncolytic viruses can be used as cancer vaccines to boost T-cell responses targeted against tumors. They can also be equipped with immune-stimulating molecules to amplify their ability to activate the immune system. Combining oncolytic viruses with other immunotherapies like checkpoint inhibitors shows significant synergy in enhancing immune responses against cancer.
Oncolytic viruses can be utilized alone as treatments or in conjunction with other approaches like chemotherapy, radiation therapy, or immunotherapy to improve therapeutic effectiveness. Chemotherapy differs from surgical removal or radiotherapy by exerting its treatment effect systemically, which is crucial for treating cancer that has spread. Combining chemotherapy with oncolytic viruses, which require targeted delivery for effective antitumor impact, is common. However, oncolytic virus clinical trials have shown that systemically administered OVs often provide suboptimal therapeutic benefits, mainly due to challenges in delivering OVs to metastatic sites. Therefore, the use of chemotherapy as a systemic complement to localized OV therapy is an actively researched topic of clinical interest.
The Impact of Oncolytic Viruses on Cancer Detection and Treatment
Oncolytic viruses represent a promising frontier in the battle against cancer, leveraging the unique ability of certain viruses to selectively infect and destroy cancer cells while sparing healthy tissues. This targeted approach not only holds the potential for more effective cancer treatment but also for enhancing detection methods. By engineering viruses to express fluorescent markers or specific imaging agents, researchers can improve the accuracy of cancer detection through non-invasive imaging techniques. This dual role of oncolytic viruses in both treatment and detection marks a significant shift towards personalized and precise medicine, where therapies can be tailored based on individual cancer characteristics, ultimately improving patient outcomes and survival rates.
Furthermore, oncolytic viruses have shown promise in overcoming some of the challenges posed by traditional cancer therapies, such as resistance and systemic toxicity. Their ability to stimulate the immune system, known as immunogenic cell death, can potentially enhance the body’s natural defenses against cancer cells. This immunostimulatory effect not only aids in directly attacking tumors but also primes the immune system to recognize and eliminate cancerous cells throughout the body, offering a systemic approach to cancer treatment. As research continues to refine the design and application of oncolytic viruses, the integration of these innovative therapies into mainstream oncology holds great promise for revolutionizing cancer treatment strategies shortly.
Oncolytic Virus Cancer Therapy Market Dynamics
With cancer remaining a top cause of death globally, there’s an increasing need for new therapies to effectively target and eliminate cancer cells. Oncolytic viruses, which can selectively infect and replicate within tumor cells, have become a promising area of cancer treatment. Oncolytic virus market growth is being driven by rising research and development efforts, increased investments from pharmaceutical companies, and a growing number of oncolytic virus clinical trials.
Currently, only three oncolytic virus therapies are approved, namely, Oncorine (Shanghai Sunway Biotech) approved in China in 2005, IMLYGIC (Talimogene laherparepvec/T-VEC; Amgen) approved in the US, Europe, and Japan in 2015, and DELYTACT (teserpaturev/G47∆; Daiichi Sankyo) approved in Japan in 2021. In addition, oncolytic virus cancer therapy is only approved for melanoma and malignant glioma (in Japan). However, the oncolytic virus cancer therapy pipeline suggests its emergence as a potential treatment for various cancers, including ovarian cancer, non-small cell lung cancer, breast cancer, renal cell carcinoma, and others.
The pipeline for oncolytic viruses is robust, with major pharmaceutical companies such as Targovax, Replimune, Genelux Corporation, Candel Therapeutics, DNAtrix, SillaJen, Treovir, Lokon Pharma AB, Istari Oncology, CG Oncology, Amgen, Daiichi Sankyo, and others actively engaged in research to enhance cancer treatment options.
With ongoing advancements and strategic collaborations, oncolytic viruses are positioned to play a pivotal role in the future of oncology, providing patients with new hope and facilitating the development of more effective and personalized cancer therapies.
Oncolytic Viruses Cancer Therapy: What Lies Ahead?
Over the next decade, the field of oncolytic virus therapies holds significant promise for the oncology industry. Treatments using oncolytic virotherapy, especially for brain cancer, skin cancer, prostate cancer, and other types, have begun to achieve major therapeutic milestones due to recent advancements.
Oncolytic viruses are a promising frontier in cancer treatment, leveraging natural agents to target and eliminate malignant cells. These viruses naturally prefer to infiltrate, replicate within, and destroy cancer cells while leaving normal tissue unharmed.
Whether naturally occurring or engineered for greater effectiveness, oncolytic viruses exhibit impressive tumor selectivity, exploiting the unique vulnerabilities of cancer cells to induce their destruction. The therapeutic strategy of oncolytic virus therapy relies on a dual mechanism: targeting tumor cells for infection, followed by inducing potent antitumor responses through the release of tumor antigens and stimulation of the host immune system. This involves the replication of oncolytic viruses within tumor cells, leading to their lysis and the release of progeny virions, which can infect adjacent cancer cells, thus amplifying the cycle of cancer cell death.
The future of oncolytic virus therapy appears bright but not without challenges that need addressing. Key areas of focus include improving delivery methods to ensure the viruses reach the tumor site effectively, overcoming the body’s immune defenses that might neutralize the therapeutic virus before it can act, and combining oncolytic virotherapy with other treatments such as immunotherapy, chemotherapy, or radiation for enhanced efficacy. Advancements in genetic engineering and a deeper understanding of tumor biology will likely play crucial roles in overcoming these hurdles. As oncolytic virus clinical trials progress and more oncolytic viruses gain regulatory approval, this innovative therapy has the potential to become a cornerstone in the fight against cancer.

Downloads
Article in PDF
Recent Articles
- Is Gene Therapy the Next Cancer Treatment Revolution?
- 8 Late and Mid Stages Adenovirus-associated Oncolytic Virus Therapies To Watch Out
- Top 7 Emerging Vaccinia Virus-associated Oncolytic Virus Therapies
- 15 Prominent Indications for Oncolytic Virus Therapy
- 7 HSV-associated Oncolytic Virus Therapies in Late and Mid-Stage