Late-Stage Thyroid Eye Disease Treatments: 4 Prominent Therapies to Consider
Jul 29, 2024
With an estimated 1 million patients affected in the US, thyroid eye disease is a burden for some disorders which become sight-threatening in upto 5% of the cases. Until today, only one thyroid eye disease treatment drug for adults has been approved by the FDA.
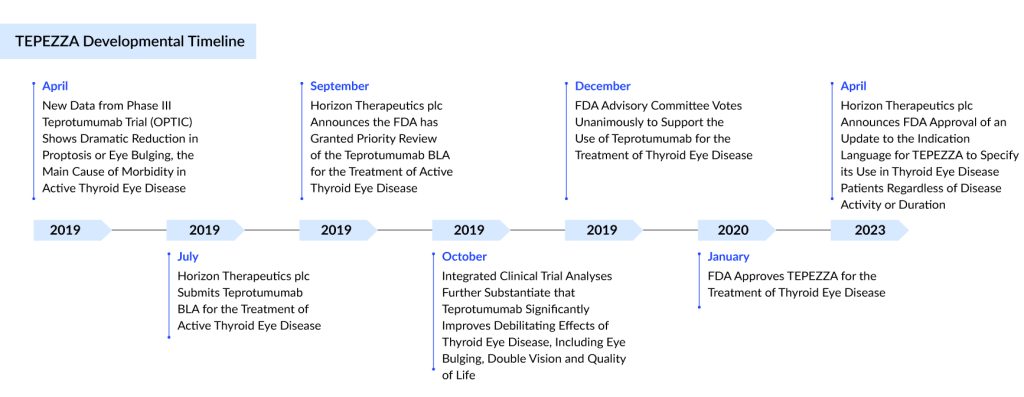
Developed by Horizon Therapeutics (now acquired by Amgen), TEPEZZA (teprotumumab), an insulin-like growth factor-1 receptor inhibitor (IGF-1R), is a fully human IgG1 monoclonal antibody produced in Chinese hamster ovary (CHO-DG44) cells with a molecular weight of approximately 148 KD.
TEPEZZA is approved for thyroid eye disease treatment in the US, Brazil, and Saudi Arabia. It is given to patients via an intravenous (IV) infusion every three weeks, totaling eight infusions over approximately five months.
Downloads
Article in PDF
Recent Articles
- Thyroid awareness month
- Thyroid Eye Disease: The Hidden Impact of Thyroid Dysfunction on Eye Health
- FDA Approves Xolair for Food Allergies; FDA Accelerated Approval for Iovance’s AMTAGVI; Astellas ...
- Graves’ Ophthalmopathy Treatment Market: A Billion-Dollar Opportunity For Pharma Companies
- Graves’ Disease Drug Pipeline: 6 Late-Stage Therapies to Watch
Teprotumumab FDA approval has emerged as a preferred moderate to severe thyroid eye disease treatment, surpassing orbital decompression as the leading option for Graves’ ophthalmopathy treatment. In its first year on the market, thyroid eye disease medication TEPEZZA experienced a 103% year-over-year growth.
Amgen’s TEPEZZA, one of the most successful launches for a rare indication, generated revenue of ~USD 2 billion in 2023 after becoming the first launched therapy for thyroid eye disease treatment in the year 2020. In January 2024, the company announced the filing in Japan, and is expected to be launched by 2025.
In April 2024, Amgen announced that it would soon submit a Marketing Authorization Application (MAA) for teprotumumab to the European Medicines Agency (EMA). This application is supported by several well-conducted thyroid eye disease clinical trials, including a Phase II study (NCT01868997), the Phase III OPTIC trial (NCT03298867), a Phase IV study (NCT04583735), and a Phase III trial in Japan (OPTIC-J, jRCT2031210453).
These studies demonstrate statistically significant and clinically relevant improvements in various aspects of thyroid eye disease, such as proptosis and diplopia, based on data from 287 patients. The trials also evaluated thyroid eye disease symptoms including pain, inflammation, redness, and functional vision, with notable clinical improvements in proptosis observed as early as six weeks into the 24-week treatment period. Teprotumumab for thyroid-associated ophthalmopathy is recognized for its established safety profile.
In March 2024, Amgen filed a marketing authorization application with the Medicines and Healthcare products Regulatory Agency (MHRA) in Great Britain, a New Drug Submission (NDS) with Health Canada, and an application with the Therapeutic Goods Administration (TGA) in Australia for teprotumumab.
The field of Graves’ disease ophthalmopathy treatment is starting to advance. New medications for thyroid eye disease treatment, which could offer long-lasting benefits, are anticipated to drive significant growth in the treatment market for the condition.
The drug candidates from major thyroid eye disease companies, including Immunovant Sciences (batoclimab), Novartis Pharmaceuticals (secukinumab), Viridian Therapeutics (VRDN-001), and others, are in advanced stages of clinical development and have the potential to impact the Graves’ ophthalmopathy treatment space greatly.
Let’s take a closer look into these late-stage therapies for thyroid eye disease treatment in detail.
Viridian Therapeutics’ VRDN-001
VRDN-001 MoA: Insulin-like growth factor-I receptor antagonists
VRDN-001 is a specialized monoclonal antibody designed to target the insulin-like growth factor-1 receptor (IGF-1R), a proven target for thyroid eye disease treatment. Currently, two major Phase III thyroid eye disease clinical trials, THRIVE and THRIVE-2, are enrolling patients with active and chronic thyroid eye disease, respectively.
Results from the THRIVE trial are anticipated by mid-year, while THRIVE-2 results are expected by the end of the year. Additionally, the company is developing another version of the drug, VRDN-003, for subcutaneous administration and plans to start a global pivotal program for it around mid-year, with trials planned for both active and chronic thyroid eye disease patients, subject to regulatory approval.
Roche’s ENSPRYNG
ENSPRYNG MoA: Interleukin 6 receptor antagonists
ENSPRYNG (satralizumab) is a humanized monoclonal antibody designed to target the interleukin-6 (IL-6) receptor. IL-6 is a signaling protein produced by immune cells in the body. The Graves’ ophthalmopathy treatment drug is currently undergoing Phase III clinical trials, which began in the fourth quarter of 2023, and according to the company’s thyroid eye disease pipeline, a filing is expected in 2025.

Immunovant’s Batoclimab
Batoclimab MoA: Neonatal Fc receptor antagonists
Immunovant’s batoclimab is a novel, fully human monoclonal antibody designed to target the neonatal Fc receptor (FcRn). In both preclinical studies and clinical trials, batoclimab has been shown to lower IgG antibody levels. Since elevated pathogenic IgG antibodies contribute to several autoimmune diseases, this candidate could potentially treat various IgG-mediated autoimmune disorders through a self-administered subcutaneous injection. The Phase III clinical trial results for thyroid eye disease treatment are anticipated and expected in the first half of 2025.
Novartis’ COSENTYX
COSENTYX MoA: IL17A protein inhibitors
COSENTYX (secukinumab) is an immunosuppressive drug available in a freeze-dried powder form and as a solution in prefilled syringes or pens for subcutaneous injection. It is a high-affinity, fully human monoclonal antibody targeting IL-17A. The drug is approved for treating moderate-to-severe plaque psoriasis, psoriatic arthritis, and axial spondyloarthritis. It is currently undergoing a Phase III trial (NCT04737330) to assess its effectiveness and safety in adult patients with active, moderate-to-severe thyroid eye disease.
Aside from these thyroid eye disease treatment drugs, several other companies are also working with their lead assets. The other drugs in the thyroid eye disease pipeline include linsitinib (Sling Therapeutics), aflibercept (Regeneron Pharmaceuticals), VB421 (ValenzaBio), and others. The launch of emerging therapies for thyroid eye disease treatment is expected to boost the therapeutic segment in the future.
In addition, the thyroid eye disease treatment drug market is anticipated to receive several new approvals soon. DelveInsight projects that the thyroid eye disease treatment market, valued at around USD 2.5 billion in 2023, will experience substantial growth by 2034. According to the estimates, about 98% of this revenue is expected to come from TEPEZZA sales in the US.
Furthermore, these emerging therapies from various pharmaceutical companies are poised to challenge Amgen’s market leader, TEPEZZA, in the treatment for thyroid eye disease. These novel treatments for thyroid eye disease leverage cutting-edge approaches, such as targeted biologics and innovative small molecules, which offer potential advantages in efficacy, safety, and patient convenience. For instance, some new thyroid eye disease therapies focus on specific inflammatory pathways or utilize advanced delivery systems to improve therapeutic outcomes.
As these alternatives advance through Graves’ ophthalmopathy clinical trials and gain regulatory approval, they may provide competitive options for patients and healthcare providers, potentially impacting TEPEZZA’s thyroid eye disease dominant position. The entry of these Graves’ ophthalmopathy therapies into the market is expected to stimulate greater innovation and improve the overall standard of care for thyroid eye disease, intensifying competition in this evolving therapeutic landscape.

Downloads
Article in PDF
Recent Articles
- Tepezza receives approval; a new way to treat Alzheimer’s
- Thyroid eye disease market: New therapies enter the TED market
- Graves’ Disease Drug Pipeline: 6 Late-Stage Therapies to Watch
- Graves’ Ophthalmopathy Treatment Market: A Billion-Dollar Opportunity For Pharma Companies
- Graves’ Disease Treatment Revolution: What’s Next in Line?