8 Late and Mid Stages Adenovirus-associated Oncolytic Virus Therapies To Watch Out
Aug 07, 2024
Table of Contents
Adenovirus-associated oncolytic virus therapies represent a promising approach to cancer treatment by harnessing the inherent ability of viruses to selectively infect and destroy cancer cells. These therapies utilize genetically modified adenoviruses, which are engineered to target and replicate specifically within tumor cells, leading to their destruction. The modifications often include alterations to the viral genome that enhance its ability to induce a potent immune response against the tumor, while minimizing harm to normal, healthy tissues. This targeted approach not only aims to directly lyse cancer cells but also helps to stimulate a broader anti-tumor immune response, potentially leading to long-lasting cancer immunity.
Moreover, adenovirus-associated oncolytic virus therapies offer the potential for combination with other therapeutic modalities, such as chemotherapy, radiation, or immune checkpoint inhibitors. This combination strategy can amplify the overall effectiveness of cancer treatment by exploiting the synergistic effects of oncolytic virotherapy and traditional therapies.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Is Gene Therapy the Next Cancer Treatment Revolution?
- 7 HSV-associated Oncolytic Virus Therapies in Late and Mid-Stage
- Oncolytic Viruses: Can Be The Next Frontier in Cancer Immunotherapy?
- Oncolytic Virus Cancer Therapy: Transforming Cancer Treatment
- 15 Prominent Indications for Oncolytic Virus Therapy
Approved Adenovirus-associated Oncolytic Virus Therapy
Currently, only one adenovirus-associated oncolytic virus therapy has been approved, namely Oncorine. Shanghai Sunway Biotech’s Oncorine is a genetically engineered adenovirus known as H101 (E1B-deletion), developed by Shanghai Sunway Biotech in China. It is used alongside chemotherapy for treating nasopharyngeal carcinoma and head and neck cancers.
The approval and successful use of Oncorine have significantly advanced the field of cancer immunotherapy involving oncolytic viruses. Also called Recombinant Human Adenovirus Type 5 Injection, Oncorine became the first oncolytic virus approved by the Chinese State Food and Drug Administration (CFDA) for cancer treatment in November 2005. Since its introduction, Oncorine has demonstrated a favorable safety profile and notable effectiveness against various tumors and malignant pleural effusion.
8 Promising Adenovirus-associated Oncolytic Virus Therapies
After the success of Oncorine adenovirus-associated oncolytic virus therapy, many pharma companies such as AmunBio, GeneMedicine, CG Oncology, Candel Therapeutics, Oncolys Biopharma, EpicentRx, Theriva Biologics, Lokon Pharma, DNAtrix, Surv BioPharma Inc., Beijing Bio-Targeting Therapeutics Technology Co., Ltd, Apices Soluciones, Calidi Biotherapeutics, ORCA Therapeutics, GeneMedicine, Akamis Bio, Valo Therapeutics, TILT Biotherapeutics, Shanghai Yuansong Biotechnology, Memgen, Akamis Bio, and others, have started working on their lead assets.
Presently, many adenovirus-associated oncolytic virus therapies are in the pipeline in various stages of development. Let’s dive deep into the most promising adenovirus-associated oncolytic virus therapies that are in the late and mid stages of development.
CG Oncology’s Cretostimogene grenadenorepvec
Phase III (Bladder Cancer)
Cretostimogene grenadenorepvec adenovirus-associated oncolytic virus therapy is an experimental oncolytic immunotherapy delivered directly into the bladder, currently undergoing evaluation in the BOND-003 Phase III clinical trial for patients with BCG-unresponsive NMIBC. This therapy is designed to specifically replicate in cells with defects in the retinoblastoma (Rb) gene pathway, which are common in urothelial carcinomas, and to stimulate an anti-tumor immune response. It enters the tumor by attaching to Coxsackievirus and Adenovirus Receptors (CAR) located in specialized intracellular and tight junctions of polarized epithelial cells.
In January 2024, the FDA awarded Fast Track and Breakthrough Therapy designations to CG0070 adenovirus-associated oncolytic virus therapy as a potential treatment for patients with high-risk NMIBC who are unresponsive to BCG therapy, including those with carcinoma in situ and either Ta or T1 tumors.
Candel Therapeutics’ CAN-2409
Phase III (Prostate Cancer)
CAN-2409 adenovirus-associated oncolytic virus therapy is a research-based, off-the-shelf adenovirus that cannot replicate and is engineered to deliver the herpes simplex virus thymidine kinase (HSV-tk) gene directly to a patient’s specific tumor. This aims to trigger a tailored, systemic immune response against the cancer. It shows promise for treating a wide range of solid tumors. Preclinical and clinical studies have demonstrated its potential both as a standalone treatment and in combination with standard therapies like radiotherapy, surgery, chemotherapy, and immune checkpoint inhibitors. CAN-2409 is developed using Candel’s enLIGHTEN™ Discovery Platform, which utilizes human biology and advanced analytics to create innovative viral immunotherapies for solid tumors. Currently, CAN-2409 adenovirus-associated oncolytic virus therapy is in Phase III oncolytic virus clinical trials for prostate cancer.
In April 2024, Candel Therapeutics, Inc. revealed that the FDA has awarded an Orphan Drug Designation to CAN-2409 for treating pancreatic cancer. Earlier, in December 2023, the company announced that the FDA had also granted Fast Track Designation to their primary investigational adenovirus product, CAN-2409, in combination with the prodrug valacyclovir, for improving overall survival in patients with pancreatic ductal adenocarcinoma (PDAC).
Oncolys Biopharma’s OBP-301
Phase II (Adenocarcinoma; Gastric Cancer; Head and Neck Cancer; Esophageal Cancer)
Telomelysin (OBP-301) adenovirus-associated oncolytic virus therapy is a genetically modified oncolytic adenovirus designed to selectively replicate within cancer cells by incorporating the human telomerase reverse transcriptase (hTERT) promoter. On June 2, 2020, Oncolys BioPharma Inc. reported that the FDA granted orphan-drug designation to OBP-301, an oncolytic viral immunotherapy, for the treatment of esophageal cancer.
Oncolytic adenoviruses hold significant promise for cancer oncolytic virus immunotherapy due to their ability to induce strong immune responses through viral replication. This replication causes tumor cells to break apart, releasing tumor antigens and generating additional immune signals. Results from a Phase I clinical trial in the US indicated that Telomelysin could trigger an abscopal effect, where both the injected and non-injected tumors shrank in melanoma patients after a single injection into one tumor. This effect was associated with increased infiltration of CD8+ T cells and antigen-presenting cells, and a decrease in Treg cells at the injection site. The OBP-301 adenovirus-associated oncolytic virus therapy is currently in Phase II trials for treating adenocarcinoma, gastric cancer, head and neck cancer, and esophageal cancer. It is also under evaluation for hepatocellular carcinoma.
In April 2024, Oncolys Biopharma began a Phase II clinical trial to evaluate the effects of combining OBP-301 with Pembrolizumab in patients with esophagogastric adenocarcinoma that has not responded to other immunotherapies. This study aims to assess the efficacy of the investigational drug, OBP-301, alongside pembrolizumab for advanced or metastatic gastric or gastroesophageal junction (GEJ) cancer.
EpicentRx’s AdAPT-001
Phase II (Sarcoma)
AdAPT-001 adenovirus-associated oncolytic virus therapy is an adenovirus engineered to produce a TGF-β “trap” that targets and neutralizes the TGF-β cytokine, aiming to enhance patient outcomes. Early results from a Phase I/II trial, both with AdAPT-001 alone and in combination with checkpoint inhibitors, indicate that AdAPT-001 is well-tolerated and shows signs of both direct and abscopal tumor responses. The observed antitumor effects in tumors resistant to checkpoint inhibitors suggest a potential synergistic mechanism between the two treatments.
Recently at ASCO 2024, Dr. Anthony P. Conley, a Sarcoma Specialist and Lead PI at MD Anderson Cancer Center (MDACC), delivered an oral presentation. The presentation focussed on the remarkable clinical activity observed with AdAPT-001 combined with an immune checkpoint inhibitor (ICI) in the ongoing Phase II open-label BETA PRIME trial, which has enrolled nearly 70 patients to date. This activity includes complete responses and several durable partial responses in tumor types resistant to checkpoint inhibitors, such as sarcoma and triple-negative breast cancer, with some patients receiving treatment for nearly two years.
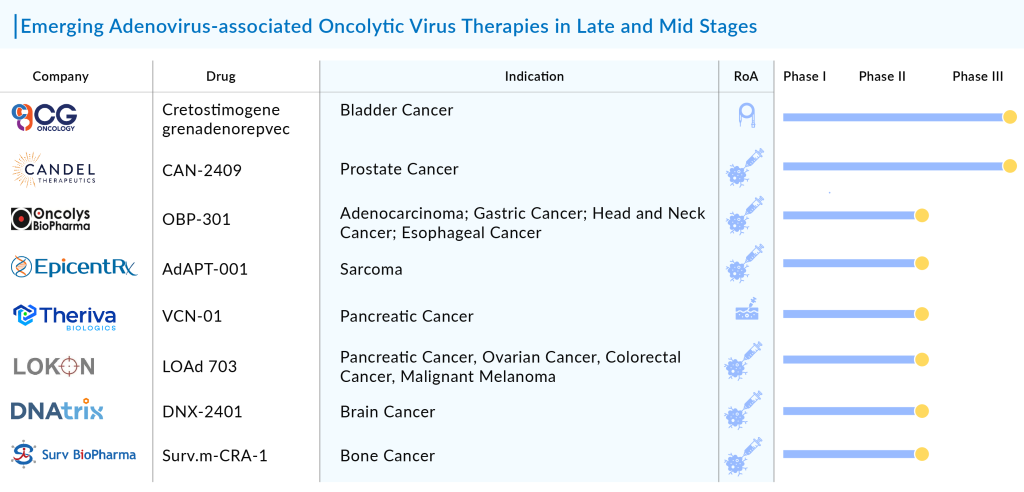
Theriva Biologics’ VCN-01
Phase II (Pancreatic Cancer)
VCN-01 adenovirus-associated oncolytic virus therapy is an oncolytic adenovirus administered systemically, designed to target and replicate within tumor cells while breaking down the tumor stroma that hinders cancer treatment. This approach allows VCN-01 to deliver several antitumor effects: (i) it specifically infects and destroys tumor cells; (ii) it improves the distribution and effectiveness of concurrent chemotherapy; and (iii) it enhances the tumor’s visibility to the immune system and boosts the impact of accompanying immunotherapy. By being administered systemically, VCN-01 can act on both primary tumors and metastatic sites. It is currently in Phase II oncolytic virus clinical trials for treating pancreatic adenocarcinoma.
In June 2023, the FDA awarded Orphan Drug Designation to VCN-01 adenovirus-associated oncolytic virus therapy. Previously, in 2011, the European Medicines Agency (EMA) had also granted Orphan Drug Designation to VCN-01 for treating PDAC.
In February 2024, Theriva Biologics revealed that the Independent Data Monitoring Committee (IDMC) advised continuing with the planned enrollment for VIRAGE. This is a multinational, Phase 2b, randomized, open-label, controlled clinical trial assessing the use of VCN-01 alongside standard chemotherapy (gemcitabine/nab-paclitaxel) as a first-line treatment for patients with metastatic pancreatic ductal adenocarcinoma.
Lokon Pharma’s LOAd 703
Phase II (Pancreatic Cancer, Ovarian Cancer, Colorectal Cancer, Malignant Melanoma)
LOAd 703 adenovirus-associated oncolytic virus therapy is an oncolytic virus under investigation in clinical trials as a potential treatment for various solid tumors. This adenovirus, or “common cold” virus, has been engineered to target and replicate within cancer cells while sparing healthy cells. Developed by Lokon Pharma, LOAd 703 adenovirus-associated oncolytic virus therapy has demonstrated encouraging anti-tumor effects in experimental models. Its mechanism involves altering the tumor microenvironment and simultaneously stimulating the immune system to attack cancer cells. In May 2015, LOAd703 received an Orphan Drug Designation from both the EMA and the FDA for its use in treating pancreatic cancer. The drug is currently in Phase II development for treating pancreatic cancer.
DNAtrix’s DNX-2401
Phase II (Brain Cancer)
DNX-2401 adenovirus-associated oncolytic virus therapy represents the culmination of more than ten years of research and is the first oncolytic virus capable of targeting and killing tumor cells with any defect in the retinoblastoma (Rb) pathway. Unlike other treatments, DNX-2401 replicates within and destroys tumor cells with disrupted growth control due to Rb pathway defects, without targeting a specific point in the pathway. This approach significantly reduces the risk of drug resistance and toxicity.
In July 2016, the European Medicines Agency awarded DNX-2401 the Priority Medicines (PRIME) designation for treating recurrent glioblastoma. Earlier, in October 2014, DNAtrix, Inc. revealed that the FDA had granted Orphan Drug Designation to adenovirus-associated oncolytic virus therapy DNX-2401 for malignant glioma. Furthermore, in June 2014, the FDA gave Fast Track status to DNX-2401 for treating patients with recurrent glioblastoma.
Currently, DNX-2401 is being tested in oncolytic virus clinical trials at the University of Texas M.D. Anderson Cancer Center for treating glioblastoma multiforme, a stage IV brain tumor. The DNX-2401 adenovirus-associated oncolytic virus therapy is now in Phase II development for this condition.
Surv BioPharma Inc.’s Surv.m-CRA-1
Phase II (Bone Cancer)
Surv.m-CRA-1, an oncolytic adenovirus developed by Surv BioPharma Inc., is designed to treat malignant bone tumors. In 2015, this drug candidate was chosen for the “Practical Research for Innovative Cancer Control” grant program by the Japan Agency for Medical Research and Development (AMED). The oncolytic virus immunotherapy drug is currently in Phase II development for treating bone tumors.
What’s Ahead for Adenovirus-associated Oncolytic Virus Therapies?
The future of adenovirus-associated oncolytic virus therapies looks promising as advancements in genetic engineering and virology continue to enhance their efficacy and safety. Apart from the promising adenovirus-associated oncolytic virus therapies mentioned above, there are several other therapies also that are in different stages of development.
The other adenovirus-associated oncolytic virus therapies in the pipeline include BioTTT001 (Beijing Bio-Targeting Therapeutics Technology Co., Ltd), AloCelyvir (Apices Soluciones), CLD-101 (Calidi Biotherapeutics), ORCA 010 (ORCA Therapeutics), GM 103 (GeneMedicine), Enadenotucirev (Akamis Bio), PeptiCRAd-1 (Valo Therapeutics), TILT-123 (TILT Biotherapeutics), YSCH-01 (Shanghai Yuansong Biotechnology), MEM-288 (Memgen), NG-350A (Akamis Bio), AMUN-003 (AmunBio), and GM102 (GeneMedicine).
All these therapies are currently in the early phases of development. The anticipated launch of these adenovirus-associated oncolytic virus therapies will boost the oncolytic virus cancer therapy market in the coming years.

Downloads
Article in PDF
Recent Articles
- Is Gene Therapy the Next Cancer Treatment Revolution?
- 7 HSV-associated Oncolytic Virus Therapies in Late and Mid-Stage
- 15 Prominent Indications for Oncolytic Virus Therapy
- Top 7 Emerging Vaccinia Virus-associated Oncolytic Virus Therapies
- Oncolytic Virus Cancer Therapy: Transforming Cancer Treatment