Exploring Growth Opportunities in the Hypophosphatasia Treatment Market: Navigating the Ultra-rare Disease Landscape
Aug 23, 2024
Table of Contents
Quick Facts
- According to DelveInsight’s analysis, in 2023, the total diagnosed prevalent cases of hypophosphatasia, an ultra-rare disease, was approximately 6,200 in the 7MM. While almost 85% have less severe forms of the disease, severity may vary based on genetic and environmental factors.
- Due to the low number of diagnosed cases, few companies have shown interest in developing treatments for this condition. To date, only one drug, STRENSIQ, has been approved. However, a few others are in clinical trials, including Alexion’s, a subsidiary of AstraZeneca, ALXN1850 (efzimfotase alfa), AM-Pharma’s Ilofotase alfa, and PuREC’s REC-01.
- Historically, developing drugs for rare or ultra-rare diseases was considered impractical due to the limited patient pool. However, this perspective has shifted in recent years, with several rare disease therapies, including STRENSIQ, proving to be blockbuster hypophosphatasia drugs with revenues exceeding USD 1 billion. In 2023, STRENSIQ alone generated USD 1.1 billion in sales, marking a growth of over 20% from the previous year.
- According to DelveInsight’s estimates, the total market size of hypophosphatasia in the 7MM was close to USD 1 billion in 2023 and is projected to grow at a CAGR of 7.2% over the forecast period (2024–2034), driven by the anticipated launch of emerging therapies, such as ALXN1850 (efzimfotase alfa), and increased awareness of the disease.
- The primary unmet needs, such as the lack of curative and affordable therapies, universal treatment guidelines, and standardized diagnostic criteria, represent major barriers. Addressing these issues would facilitate market expansion and improve disease management.
Hypophosphatasia: Disease Background and Overview
Hypophosphatasia is an inborn error of metabolism characterized by low serum alkaline phosphatase activity (hypophosphatasemia), resulting from loss-of-function mutations in the ALPL gene, which encodes the tissue-nonspecific alkaline phosphatase (TNSALP). The TNSALP protein, linked to the cell membrane via a glycosylphosphatidylinositol anchor, hydrolyzes phosphate compounds like inorganic pyrophosphate. This enzyme is crucial for the mineralization of hard tissues.
Hypophosphatasia is classified into six forms, each presenting distinct symptoms depending on severity: perinatal, perinatal benign, infantile, childhood, adult, and odontohypophosphatasia. The perinatal form is the most severe and is often diagnosed within the first year of life, with a mortality rate of up to 50%. In contrast, odontohypophosphatasia is the mildest form, leading to premature loss of fully rooted primary teeth and/or severe dental caries. Misdiagnosis is common in milder forms, as they typically occur in adults and are often not linked to skeletal abnormalities, a major feature of hypophosphatasia.
Downloads
Article in PDF
Recent Articles
“Adults with hypophosphatasia may often be unaware of their condition. They might experience joint discomfort or have a history of fractures that haven’t healed correctly. Some individuals may not show any symptoms at all until routine tests reveal elevated levels of vitamin B6 or decreased alkaline phosphatase activity.”
Northwestern University, US
Until recently, the management of hypophosphatasia was predominantly symptomatic and supportive. Severely affected neonates often require ventilation, which can be technically challenging. Neonatal seizures, while treatable with Vitamin B6, may become refractory.
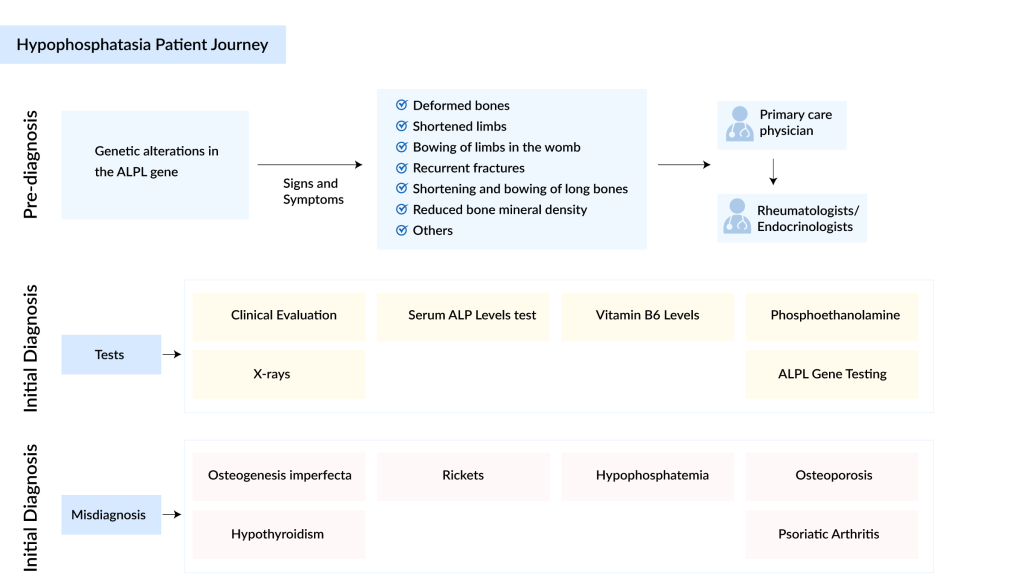
Effective management of hypophosphatasia necessitates a multidisciplinary approach to ensure optimal patient care and family support. Pediatric patients require the expertise of pediatricians, whereas older patients benefit from the care of adult physicians specializing in metabolic bone diseases, such as rheumatologists and endocrinologists. Given the rarity of hypophosphatasia, optimal care is often found in specialist centers where teams have extensive experience with the condition. For geographic constraints, a ‘hub and spoke model’ can be implemented, where local teams receive guidance from expert teams remotely. Orthopedic surgeons, dentists with a specific interest in hypophosphatasia, and pain management specialists are also crucial members of the care team. Specialist nurses provide essential support, information, and assistance in accessing appropriate care. Psychological support, either through formal psychological services or informally through specialist nurses, is often necessary. Additionally, physiotherapy and occupational therapy may be required to address physical limitations.
In 2015, the US FDA approved STRENSIQ (asfotase alfa) as the first medical hypophosphatasia treatment for perinatal, infantile, and juvenile-onset hypophosphatasia. In the US, patients of any age with pediatric-onset hypophosphatasia are eligible for this bone-targeted TNSALP replacement therapy given by subcutaneous injection.
Other therapies used in the hypophosphatasia treatment include non-steroidal anti-inflammatory drugs (NSAIDs) like ibuprofen, acetaminophen, and others, Vitamin D3, Phosphate Binders (lanthanum carbonate and others), etc.
Hypophosphatasia Treatment Challenges
AstraZeneca’s STRENSIQ, the only approved hypophosphatasia therapy, is recommended for treating perinatal, infantile, and juvenile-onset hypophosphatasia. The majority of patients, particularly adults, still lack treatment options. The annual cost of STRENSIQ therapy varies significantly depending on the patient’s weight, as the recommended dose is 2 mg/kg three times a week or 1 mg/kg six times a week. According to DelveInsight’s estimates, the annual cost of treatment (ACoT) starts at approximately USD 290,000 for patients weighing between 0 kg and 9 kg. It can reach up to USD 2.5 million for patients weighing more than 40 kg. On average, the annual cost of treatment (ACoT) for a 20 kg patient exceeds USD 1 million in the US, which is a significant barrier to its adoption.
Furthermore, since the disease is relatively rare, treatment is available only at specialized centers. It often requires a multidisciplinary team of specialists, including pediatricians, orthopedic surgeons, pedodontists, pain management experts, and other healthcare professionals.

Emerging Therapies to Improve Hypophosphatasia Treatment
Emerging hypophosphatasia therapies like ALXN1850 aim to enhance the bioavailability and shelf life of a drug, which can lead to reduced dosage or less frequent administration. In addition, Ilofotase alfa is being developed specifically for the adult population, which currently lacks any approved hypophosphatasia therapy. Furthermore, some innovative cell and gene therapies for hypophosphatasia treatment such as ARU-2801 and Engineered B Cell Medicines (ALP-BCM), are currently in the preclinical developmental stage.
ALXN1850 (efzimfotase alfa): AstraZeneca (Alexion Pharmaceuticals)
AstraZeneca’s ALXN1850 (efzimfotase alfa) is an enzyme replacement therapy that substitutes for deficient alkaline phosphatase (ALP) activity. It targets ALP substrates to enhance bone mineralization and alleviate systemic disease manifestations. This next-generation therapy for hypophosphatasia offers higher activity, greater bioavailability, and a longer half-life compared to STRENSIQ (asfotase alfa).
One of the main drawbacks of STRENSIQ is the frequent dosing schedule of 2 mg/kg three times a week or 1 mg/kg six times a week, which ALXN1850 (efzimfotase alfa) aims to address. In ongoing clinical trials, ALXN1850 is administered once every 2 weeks. If approved, it will be a much-needed breakthrough in hypophosphatasia treatment. According to DelveInsight’s estimates, the hypophosphatasia drug is expected to turn into a blockbuster drug by generating more than USD 1.5 billion by 2034.
Ilofotase alfa: AM Pharma
AM Pharma’s Ilofotase alfa is a proprietary recombinant alkaline phosphatase constructed from two human isoforms of alkaline phosphatase that is well tolerated, stable, and highly active in multiple clinical trials in acute kidney injury and hypophosphatasia treatment. In a recently completed Phase I/II trial, the drug showed positive results, paving the way for further development.
Additionally, the hypophosphatasia drug targets the adult population. As per estimates, adult-onset cases account for more than 50% of the total diagnosed prevalent cases of hypophosphatasia.
Unmet Needs in Hypophosphatasia Treatment
There is a significant gap in addressing hypophosphatasia, from diagnosis to treatment. The rarity of hypophosphatasia, lack of awareness among physicians, and absence of pathognomonic symptoms lead to underdiagnoses or misdiagnoses, especially for mild and moderate forms. Also, estimating the prevalence of hypophosphatasia is challenging due to the scarcity of recent, country-specific epidemiological data.
In terms of hypophosphatasia treatment, although enzyme replacement therapy and supportive measures are available, hypophosphatasia continues to be associated with high morbidity and mortality rates, particularly in children. There is a pressing need for curative therapies to serve as alternatives to current hypophosphatasia treatments. Furthermore, the high cost of existing therapies restricts their accessibility, highlighting the critical need for the development of more affordable hypophosphatasia treatment options.
Hypophosphatasia Treatment Market Outlook for the Future
The hypophosphatasia treatment market presents a unique opportunity for innovative treatments that could potentially generate significant revenue, possibly exceeding USD 1 billion. This potential is exemplified by STRENSIQ, the only approved therapy, that has achieved substantial sales despite a relatively low number of diagnosed cases. In a value-based payment system, high-cost therapies can receive reimbursement if they demonstrate significant improvements in patient symptoms and quality of life.
A significant future opportunity in the hypophosphatasia market lies in the development and commercialization of gene therapies, which could provide long-term or curative solutions for the disease. Current treatments, such as enzyme replacement therapy, mainly manage symptoms and require lifelong use. Gene therapy has the potential to transform the hypophosphatasia treatment landscape by targeting the genetic root of hypophosphatasia. This advancement could address not only severe cases but also milder forms, greatly expanding the market and meeting a crucial need for durable and effective hypophosphatasia treatment options.

Downloads
Article in PDF



