Top 7 Emerging Vaccinia Virus-associated Oncolytic Virus Therapies
Aug 28, 2024
Table of Contents
Vaccinia virus-associated oncolytic virus therapies represent a promising approach to cancer treatment, leveraging the ability of the vaccinia virus to selectively infect and destroy cancer cells. The vaccinia virus, historically used in the smallpox vaccine, has been engineered to serve as an oncolytic virus due to its robust replication capabilities and ability to induce strong immune responses. These modified viruses can be designed to preferentially target tumor cells, sparing healthy tissues, and can be armed with therapeutic genes that enhance their anticancer effects. The virus’s replication within tumor cells leads to cell lysis, which not only destroys the cancer cells directly but also stimulates the immune system to recognize and attack the tumor.
The development of vaccinia virus-based oncolytic therapies has shown promise in preclinical and early clinical trials. These therapies are particularly advantageous because of the vaccinia virus’s ability to be genetically modified, allowing for the inclusion of additional therapeutic agents such as cytokines or immune checkpoint inhibitors. Moreover, the virus can be administered systemically or directly into the tumor, offering flexibility in treatment approaches. The dual mechanism of action—direct oncolysis and immune activation—makes vaccinia virus-associated oncolytic therapies a compelling candidate for treating various cancers, including those resistant to conventional therapies.
Downloads
Article in PDF
Recent Articles
- Is Gene Therapy the Next Cancer Treatment Revolution?
- Oncolytic Virus Cancer Therapy: Transforming Cancer Treatment
- Oncolytic Viruses: Can Be The Next Frontier in Cancer Immunotherapy?
- 15 Prominent Indications for Oncolytic Virus Therapy
- 8 Late and Mid Stages Adenovirus-associated Oncolytic Virus Therapies To Watch Out
7 Most Promising Vaccinia Virus-associated Oncolytic Virus Therapies in the Pipeline
Currently, several companies including Genelux Corporation, SillaJen, BioInvent International, BioEclipse Therapeutics, Transgene, VacV Biotherapeutics, and others are developing vaccinia virus oncolytic virus therapies in various stages of development.
Let’s dive deep into the upcoming vaccinia virus-associated oncolytic virus therapies that have the potential to change the oncolytic virus immunotherapy landscape in the coming years.
Genelux Corporation’s Olvi-Vec
Phase III (Platinum-resistant Ovarian Cancer)
Olvi-Vec is a proprietary oncolytic vaccinia virus engineered to be non-pathogenic, enhancing its safety, tumor selectivity, and therapeutic potential. This virus induces immunogenic cell death through oncolysis, which in turn activates the immune system and establishes long-term immune memory for cancer immunotherapy. Over 150 patients have been treated with Olvi-Vec in oncolytic virus clinical trials, where it was generally well tolerated and showed clinical benefits. Patients who received Olvi-Vec-primed immunochemotherapy responded to platinum-based therapies, even after previously being resistant or refractory to them.
In November 2023, Genelux Corporation revealed that the FDA granted Fast Track designation to their development program for Olvi-Vec aimed at treating patients with platinum-resistant/refractory ovarian cancer. Olvi-Vec vaccinia virus-associated oncolytic virus therapy is currently in Phase III development for treating resistant or refractory ovarian cancer.
SillaJen’s PEXA-VEC
Phase II (Renal Cell Carcinoma; Prostate Cancer; Melanoma)
Pexa-Vec is SillaJen’s most advanced candidate from their proprietary SOLVE (Selective Oncolytic Vaccinia Engineering) platform. The backbone of Pexa-Vec is based on a vaccinia strain that has been safely used in millions of individuals. It has been engineered to target common genetic defects in cancer cells by removing its thymidine kinase (TK) gene, which makes Pexa-Vec reliant on the cellular TK that is consistently overexpressed in cancer cells.
Additionally, Pexa-Vec is designed to express the GM-CSF protein, which enhances the destruction of cancer cells, triggering a series of events that lead to tumor cell death, the collapse of tumor blood vessels, and a sustained immune response against the tumor. Pexa-Vec has demonstrated efficacy when administered directly into tumors or through intravenous injection.
In May 2013, Jennerex, Biotherapeutics announced that the FDA had granted orphan drug status to Pexa-Vec for the treatment of hepatocellular carcinoma. Similarly, in November 2009, the European Commission granted orphan drug designation to Sirius Regulatory Consulting Limited, UK, for the vaccinia GM-CSF/TK-deactivated virus, also for the treatment of hepatocellular carcinoma. Pexa-Vec vaccinia virus-associated oncolytic virus therapy is currently in Phase III oncolytic virus clinical trials for the treatment of hepatocellular carcinoma.
BioInvent International/Transgene’s BT 001
Phase I/I (Soft Tissue Sarcoma; Melanoma)
Co-developed by Transgene and BioInvent, BT-001 is a genetically modified virus derived from the vaccinia virus, intended for use in research treatments. By inactivating the TK and RR genes, BT-001 vaccinia virus-associated oncolytic virus therapy selectively targets rapidly dividing cells, such as cancer cells, while sparing healthy ones. Once inside the tumor cells, the virus replicates, leading to the destruction of these cells through oncolysis.
This genetic modification also reduces viral replication compared to the vaccinia strains used in the smallpox eradication campaign. Additionally, BT-001 is engineered to enhance the patient’s anti-tumor immune response by producing the anti-CTLA4 antibody (4-E03) and human GM-CSF directly at the tumor site, further contributing to tumor cell destruction.
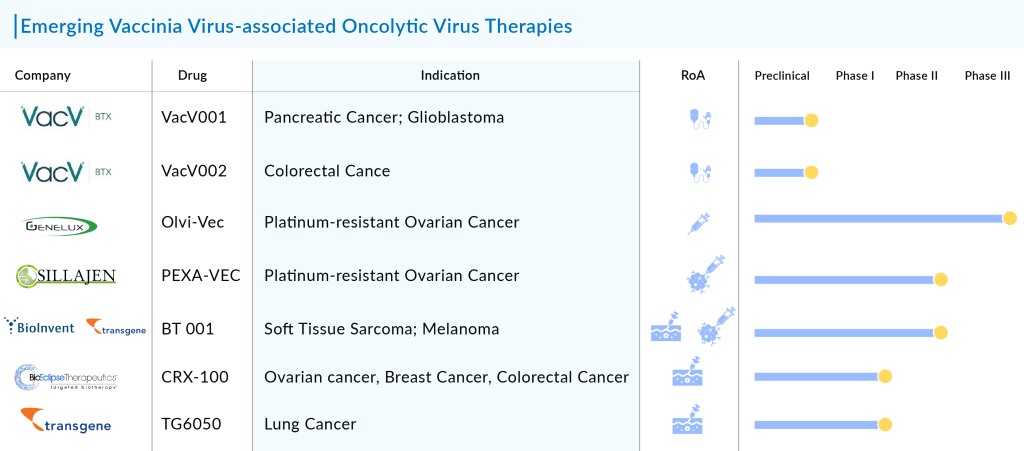
BioEclipse Therapeutics’ CRX-100
Phase I (Ovarian cancer, Breast Cancer, Colorectal Cancer)
CRX-100 is a novel, patented immunotherapy administered intravenously and designed to target solid tumors. It is being developed to offer potentially curative treatments across a wide range of tumor types, including certain rare pediatric cancers. Originating from technology exclusively licensed by Stanford University, CRX-100 aims to provide a curative option for patients with challenging malignancies, encompassing both solid and liquid tumors. This drug integrates activated immune cells with a tumor-selective oncolytic virus. Currently, CRX-100 vaccinia virus-associated oncolytic virus therapy is in Phase I clinical trials for advanced malignancies.
Transgene’s TG6050
Phase I (Lung Cancer)
TG-6050 is an oncolytic virus developed from Transgene’s Invir.IO platform. It encodes interleukin-12 (IL-12) and an anti-CTLA4 antibody, which may stimulate a strong antitumor immune response. Administered intravenously, TG-6050 vaccinia virus-associated oncolytic virus therapy is currently being tested in a Phase I clinical trial for treating patients with recurrent metastatic advanced non-small cell lung cancer.
In April 2023, Transgene began a Phase I trial to evaluate escalating doses of TG6050 delivered through intravenous infusion to patients with advanced non-small cell lung cancer. The trial’s primary objective is to establish the optimal dose and administration schedule for TG6050 by assessing the safety and tolerability of both single and repeated IV infusions at increasing doses in these patients. The study is expected to conclude by October 2024, with approximately 36 participants anticipated for enrollment.
VacV Biotherapeutics VacV001 and VacV002
VacV001: Preclinical (Pancreatic Cancer; Glioblastoma)
VacV002: Preclinical (Colorectal Cancer)
VacV Biotherapeutics, a spin-off from Queen Mary University of London, is a UK-based company that has developed a platform using oncolytic viruses to provoke lasting immune responses against tumor antigens. This platform is based on the Vaccinia virus, a poxvirus similar to smallpox, which has been genetically modified to positively influence the tumor microenvironment.
VacV’s primary candidate, VacV001 vaccinia virus-associated oncolytic virus therapy, is undergoing preclinical trials for its potential effectiveness in treating glioblastoma and pancreatic cancer, while VacV002 vaccinia virus-associated oncolytic virus therapy is being tested for colorectal cancer with liver metastasis. The VacV002 virus construct expresses the fusion gene FCU1 (ΔI4LΔJ2R/FCU1 VACV) under the control of the p11K7.5 promoter. Additionally, the VacV002 virus construct also expresses GFP (ΔI4LΔJ2R/GFP VACV), designated VVTG17990, through homologous recombination.
Additionally, studies in mice have shown that pre-treating with the PI3Kδ-selective inhibitor IC87114 improves the intravenous delivery of the vaccines, leading to better anti-tumor outcomes. The company has secured over $3 million in funding, with the most recent round completed in 2022, the year of its establishment.
What’s Ahead for Vaccinia Virus-associated Oncolytic Virus Therapies?
Vaccinia virus-associated oncolytic virus therapies represent a promising frontier in cancer treatment, capitalizing on the virus’s ability to selectively infect and destroy cancer cells while sparing normal tissue. These therapies exploit the natural characteristics of the vaccinia virus, which is highly immunogenic and has a large genomic capacity, allowing for the insertion of therapeutic genes. Recent advances have focused on enhancing the specificity and efficacy of these therapies by modifying the virus to express immune-stimulating molecules, which can boost the body’s anti-tumor response. Oncolytic virus clinical trials have shown encouraging results, with some vaccinia-based oncolytic viruses demonstrating significant tumor regression in patients with advanced cancers.
Looking ahead, the future of vaccinia virus-associated oncolytic therapies lies in refining their safety profile and expanding their application across different cancer types. Ongoing research is exploring combinations of these therapies with other treatment modalities, such as checkpoint inhibitors and radiation, to enhance overall efficacy. Furthermore, personalized approaches that tailor the virus to the individual’s tumor genetics are under investigation, potentially leading to more effective and targeted treatments. As our understanding of the tumor microenvironment and the immune system’s role in cancer progresses, vaccinia virus-associated oncolytic therapies could become a cornerstone in the fight against cancer, offering new hope for patients with difficult-to-treat malignancies.

Downloads
Article in PDF
Recent Articles
- Oncolytic Virus Cancer Therapy: Transforming Cancer Treatment
- Oncolytic Viruses: Can Be The Next Frontier in Cancer Immunotherapy?
- Is Gene Therapy the Next Cancer Treatment Revolution?
- 7 HSV-associated Oncolytic Virus Therapies in Late and Mid-Stage
- 15 Prominent Indications for Oncolytic Virus Therapy