DUPIXENT Receives First-Ever Biologic Approval for COPD: Adds Another Jewel in its Crown
Oct 07, 2024
After more than ten years without significant therapeutic advancements in chronic obstructive pulmonary disease (COPD), patients with this progressive condition now have two new treatment options, with more likely to follow.
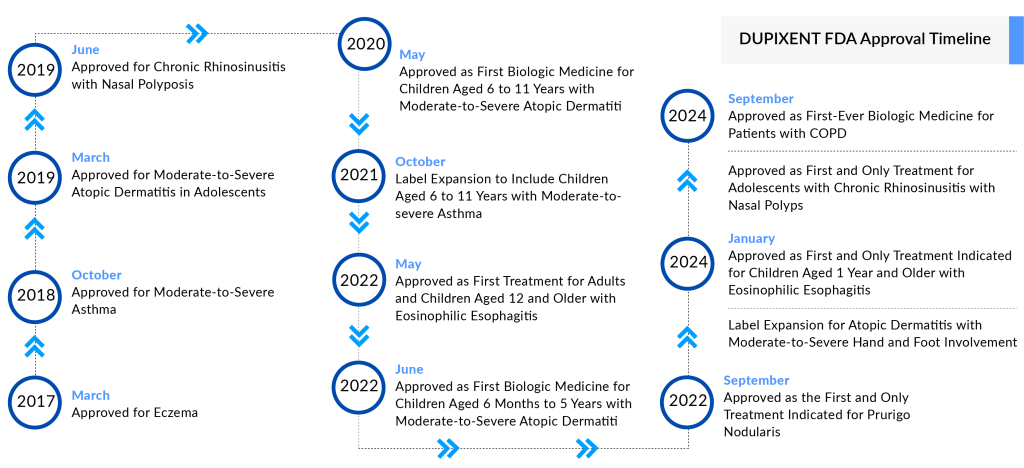
Regeneron and Sanofi recently announced that the FDA has broadened the use of the highly successful drug DUPIXENT to include the treatment of COPD. This approval increases DUPIXENT’s US indications to six, with its first approval occurring seven years ago for atopic dermatitis.
With this label expansion, DUPIXENT becomes the first biologic therapy available for COPD patients in the US. It is now approved as an add-on maintenance treatment for adults with poorly controlled COPD who have elevated blood eosinophil counts (BEC).
Downloads
Click Here To Get the Article in PDF
Recent Articles
- FDA Approves Neurocrine’s CRENESSITY for Congenital Adrenal Hyperplasia; Checkpoint’s UNLOXCYT Ap...
- Clinical
- Assessment of Key Products that Got FDA Approval in Second Half (H2) of 2021
- Ipsen’s Cabometyx Rejected by NICE; Vertex and CRISPR Therapeutics’s Submit BLA to the FDA for ex...
- Sanifit nets USD 80.9 M; FDA approves Dupixent for CRS; AbbVie buys Allergan
DUPIXENT (dupilumab) is a fully human monoclonal antibody developed by Sanofi and Regeneron to treat conditions associated with type-2 inflammation. It works by blocking the signaling pathways of interleukin-4 (IL4) and interleukin-13 (IL13) by binding to the shared IL-4Rα subunit of both receptors. Unlike immunosuppressants, DUPIXENT does not suppress the immune system. DUPIXENT is approved in more than 60 countries. Regeneron and Sanofi have been collaborating on the global development of Dupilumab since January 2018.
DUPIXENT received FDA approval for atopic dermatitis in 2017, followed by approvals for asthma in 2018, rhinosinusitis with nasal polyps in 2019, prurigo nodularis in 2022, eosinophilic esophagitis in 2024. The price of DUPIXENT has increased over the years, with a 13% rise since 2017, and in January 2024, the list price for a month’s supply rose by 6% to approximately $3,800.
Explore how DUPIXENT breaks the ground by making it the first and only eosinophilic esophagitis treatment for pediatric patients
In July 2024, the European Medicines Agency granted approval for DUPIXENT for this indication, and on the same day as the US authorities, Chinese authorities followed suit. The diagnosed prevalent population of COPD in the 7MM was around 33 million in 2023, as per DelveInsight. The estimates suggest the higher diagnosed prevalence of COPD in the United States with nearly 19 million diagnosed cases in 2023 among the other 7MM countries which is expected to increase by 2034.
The FDA’s approval is founded on data from two significant Phase III trials (BOREAS and NOTUS) that assessed the safety and efficacy of DUPIXENT versus a placebo in adults with inadequately controlled COPD, who were already receiving maximal standard inhaled therapy (with nearly all on triple therapy) and had blood eosinophil levels of ≥300 cells per μL. In the BOREAS trial (n=468) and the NOTUS trial (n=470), patients receiving Dupixent showed the following results compared to those on placebo (BOREAS n=471; NOTUS n=465):
i) A 30% and 34% decrease in the annualized rate of moderate or severe COPD exacerbations over 52 weeks, which was the primary endpoint.
ii) An improvement of 74 mL and 68 mL in post-bronchodilator FEV1 from baseline at week 12 compared to placebo, with these improvements sustained at 52 weeks. Statistically significant enhancements of similar magnitude were noted in pre-bronchodilator FEV1 from baseline at both 12 and 52 weeks, a key secondary endpoint.
iii) A 51% response in health-related quality of life measures in both trials, compared to 43% and 47% for placebo at 52 weeks, as evaluated by a 4-point improvement on the St. George’s Respiratory Questionnaire (SGRQ).
DUPIXENT stands as a beacon of hope for patients and a strong performer in the market. In 2023, DUPIXENT generated $11.6 billion in revenue, and in the first half of this year, it generated $6.1 billion in revenue.
DUPIXENT is among the new COPD medications gaining attention for the treatment of COPD. Recently, the US regulatory body approved Verona Pharma’s Ohtuvayre, a selective dual inhibitor targeting phosphodiesterase 3 (PDE3) and phosphodiesterase 4 (PDE4) enzymes. This medication uniquely facilitates airway dilation while also reducing inflammation in patients. It has been recognized for use both as a standalone treatment and as an adjunct to existing therapies.
In addition, several other companies are entering the market with potential COPD treatments. Recently, GSK announced that its leading respiratory medication, NUCALA (mepolizumab), combined with inhaled maintenance therapy, significantly reduced the frequency of moderate to severe exacerbations in COPD patients for up to two years.
Mepolizumab is currently in Phase III clinical investigation for COPD specifically characterized by frequent exacerbations and eosinophil levels. It is formulated as an injectable solution and powder for solution for SC route of administration. The IL-5 receptor complex causes the activation of multiple signaling pathways including the release of cytokines, neuromediators, chemokines, as well as several kinases that promote eosinophil differentiation, proliferation, recruitment, and degranulation.
Amgen and AstraZeneca are optimistic about their asthma medication TEZSPIRE being a possible treatment for COPD, given its proven effectiveness in patients with elevated BEC levels. Furthermore, Regeneron and Sanofi are also exploring a second COPD candidate, the IL-33 inhibitor itepekimab, which is currently undergoing two phase III trials. Late-breaking findings from the Phase IIa COURSE Trial, presented at the ATS 2024 conference, highlighted TEZSPIRE’s effect on reducing COPD exacerbations in patients with varying eosinophil levels.
AstraZeneca is also working with its candidate FASENRA and trying to bring this into the COPD market. FASENRA (benralizumab) is a humanized recombinant monoclonal antibody of the isotype IgG1k immunoglobulin that specifically binds to the alpha chain of the interleukin 5 receptor (IL-5R) expressed on eosinophils and basophils. It inhibits the binding of IL-5 as well as the hetero-oligomerization of the alpha and beta subunits of the IL-5R, thus blocking signal transduction.
Besides, it is an afucosylated IgG which gives it a high affinity for the FcγRIIIα receptor in natural killer cells, macrophages, and neutrophils. Benralizumab, FDA-approved in November 2017 for severe eosinophilic asthma, was developed by MedImmune, AstraZeneca’s global biologics research and development arm. The company is currently developing FASENRA in a Phase III clinical trial for COPD treatment.
Additionally, Sanofi’s pipeline indicates that DUPIXENT is being investigated for several new indications, including ulcerative colitis, chronic spontaneous urticaria, eosinophilic gastritis, and bullous pemphigoid, as well as for chronic pruritus in the US and Japan.
In September 2024, the FDA expanded DUPIXENT’s approval to include adolescents aged 12 to 17 with inadequately controlled CRSwNP as an add-on maintenance therapy. Additionally, the pivotal ADEPT trial for bullous pemphigoid met all primary and secondary endpoints, potentially making DUPIXENT the first targeted therapy for this condition in the US and EU.
Furthermore, the LIBERTY-CUPID Study C confirmed that DUPIXENT achieved its primary and secondary endpoints in treating uncontrolled chronic spontaneous urticaria, supporting a regulatory resubmission in the US by the end of the year. If approved, it would be the first targeted therapy for CSU in a decade.
In summary, DUPIXENT is set to continue its impressive legacy despite the influx of new drug approvals, thanks to its unmatched efficacy and expanding label. With its well-established safety profile and growing list of indications—from atopic dermatitis to asthma, and chronic rhinosinusitis to eosinophilic esophagitis—DUPIXENT has become a cornerstone therapy across multiple immunological conditions. As it continues to demonstrate life-changing results for patients, and with ongoing studies poised to unlock even more potential uses, DUPIXENT is primed to maintain its leadership in the biologic market, offering hope where other treatments fall short.

Downloads
Article in PDF
Recent Articles
- Towards a Promising Future: Unveiling Advancements in Chronic Spontaneous Urticaria (CSU) Treatment
- 6 Emerging Treg Cell-based Therapies Shaping the Future of Immunotherapy
- Airway Management Devices: Charting the Evolving Market Trends and Key Innovations
- 4 Investigational Chronic Pruritus Drugs Shaping the Treatment Landscape
- Lack of effective therapy: A major Nontuberculous Mycobacteria Infection Market Driver