12 Breakthrough Prostate Cancer Drugs in Late-Stage Development
Oct 11, 2024
Table of Contents
Prostate cancer is one of the most common cancers among men, affecting about 1 in 8 men in the United States at some point in their lives. According to estimates from DelveInsight, there were nearly 8.2 million total cases of prostate cancer in the 7MM in 2023, although this number does not indicate how many of these cases are treatable.
DelveInsight’s analysis also found that the US had 1.5 million diagnosed prevalent cases of prostate cancer in 2023, a number that is projected to rise by 2034 due to factors such as an aging population, improved screening techniques that enable earlier detection, lifestyle, and environmental changes, and advancements in medical technology that enhance diagnosis and treatment options.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Now, Nanoparticles for Cancer Treatment
- Dendreon touts; Zytiga slashes risk; Lynparza posts; Dacomitinib wins; Alecensa trounces
- J&J’s 2-in-1 Tablet for Prostate Cancer; FDA Approves TALVEY for Heavily Pretreated Multiple ...
- Gilead’s Magrolimab Plus Azacitidine for MDS; FDA Approveds VANFLYTA for Newly Diagnosed AML; FDA...
- Another Feather in the Cap for Xtandi and Keytruda — The Two Main Cancer Drugs
Did you know September is Prostate Cancer Awareness Month? Read our latest article to learn more about prostate cancer
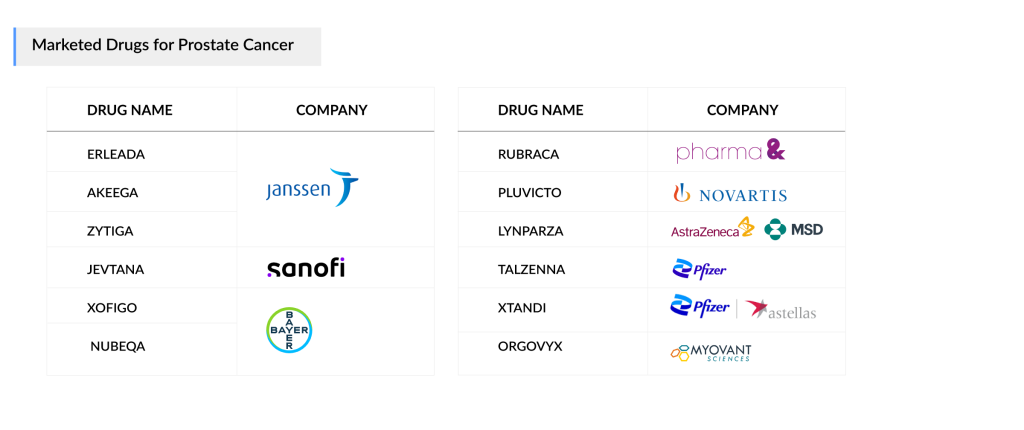
Approved Prostate Cancer Drugs
Currently approved prostate cancer drugs include XTANDI from Astellas/Pfizer and ZYTIGA from Janssen, both available for over a decade. Although ZYTIGA’s generics have been on the US market since 2019 and the EU since 2022, resulting in significant revenue losses, particularly in the US, the drug is being tested in combination with new therapies, increasing its patient base through its active ingredient, abiraterone acetate.
Janssen expanded its portfolio with ERLEADA for mHSPC in 2019, after receiving approval for nmCRPC in 2018. Bayer’s NUBEQA has also quickly gained traction as a strong competitor. PARP inhibitors are gaining ground in patients with HRR gene mutations (e.g., BRCA1/2). AstraZeneca’s LYNPARZA was approved for first-line prostate cancer treatment, and Pharma&Schwiez’s RUBRACA became available for third-line mCRPC in 2020.
In 2023, Pfizer’s TALZENNA and Janssen’s AKEEGA were approved for first-line use. Additionally, Myovant Sciences’ ORGOVYX and Sanofi’s JEVTANA have been approved for mCRPC in the US. In 2022, the mCRPC treatment market experienced the approval of Novartis’ radioligand therapy, PLUVICTO, which generated higher-than-expected revenue in the third-line mCRPC segment. Novartis plans to expand its presence into earlier-line mCRPC by 2024 and mHSPC by 2025 in the US market.
12 Promising Prostate Cancer Drugs Approaching Approval
The prostate cancer drug pipeline is buzzing with groundbreaking therapies that promise to transform treatment. With a focus on minimizing side effects and extending survival, the latest developments are designed to tackle the most aggressive forms of prostate cancer, offering new hope to patients and their families. The future of prostate cancer treatment has never looked brighter.
Key companies such as Eli Lilly and Company, Curium, Tavanta Therapeutics, Candel Therapeutics, Lantheus, Pfizer, Dongkook Pharmaceutical, Telix Pharmaceuticals, AstraZeneca, Exelixis, Jiangsu HengRui Medicine, SOTIO, AB Science, Bristol Myers Squibb, and Merck are currently the top runners with their respective candidates in late-stage of development. Let’s take a look at these late-stage prostate cancer drugs in detail
Curium’s 177Lu-PSMA-I&T
177Lu-PSMA-I&T is a radiopharmaceutical used to treat mCRPC. It targets PSMA-expressing tumor cells and destroys them using beta particle radiation. Curium continues to provide clinical trial materials and enroll patients in the ongoing Pivotal ECLIPSE trial without interruptions.
In April 2024, Curium signed an agreement with Eczacıbaşı Holding and Bozlu Group to acquire Eczacıbaşı-Monrol Nuclear Product. This acquisition is expected to enhance their complementary geographical presence, expand Lutetium-177 (Lu-177) capabilities, and strengthen PET & SPECT nuclear medicine infrastructure. It will also support the development of advanced radionuclide and radiopharmaceutical pipelines for both diagnostics and therapy.
Merck/Orion’s Opevesostat
Opevesostat is an oral, non-steroidal, and selective CYP11A1 inhibitor developed by Orion, currently under investigation for treating hormone-dependent cancers like prostate cancer. Its mechanism involves blocking CYP11A1 activity to reduce the production of steroid hormones and their precursors, which could activate the androgen receptor signaling pathway. Merck and Orion have launched two key Phase III clinical trials, OMAHA1 (NCT06136624) and OMAHA2a (NCT06136650), to evaluate opevesostat in combination with hormone replacement therapy for certain patients with mCRPC.
In July 2024, Merck and Orion revealed that they had jointly exercised options granting Merck exclusive global rights to develop and commercialize prostate cancer drug opevesostat for the treatment of mCRPC. The global license is contingent upon approval under the Hart-Scott-Rodino Antitrust Improvements Act and other standard conditions. It is anticipated to take effect in the third quarter of 2024.
AstraZeneca’s AZD5305
AZD5305 is a potent and selective oral PARP inhibitor (PARPi) that specifically targets and traps PARP1, unlike currently approved PARPis that affect both PARP1 and PARP2. Preclinical studies indicate that inhibiting PARP1 leads to antiproliferative effects, whereas inhibiting PARP2 significantly contributes to hematological toxicity. Consequently, prostate cancer drug AZD5305 could offer a better therapeutic index with reduced toxicity.
According to the clinical trial appendix from AstraZeneca’s first half of the year, the company expects to have Phase III data for saruparib (EvoPAR-Prostate01 NCT06120491) related to homologous recombination repair-mutated mCSPC by 2025 or later. Additionally, data from the Phase I/IIa trial (PETRANHA, NCT05367440) of saruparib combined with new hormonal agents for mPC is also anticipated in 2025 or later. The company is also looking forward to Phase I data (ASCERTAIN, NCT05938270) involving saruparib, darolutamide, and their combination in men with newly diagnosed prostate cancer in the first half of 2025.
Tavanta Therapeutics’ TAVT-45
TAVT-45 is a new oral suspension formulation of abiraterone acetate, developed as an alternative to branded ZYTIGA tablets, offering significant advantages. The granules of TAVT-45 dissolve quickly in water or juice, making it easier for approximately 20–30% of patients with dysphagia or swallowing difficulties to consume. Clinical studies have shown that TAVT-45 results in less variability in abiraterone exposure compared to ZYTIGA, and it has improved bioavailability when subjects are fasting. This enhanced absorption profile allows for a lower total daily dose of TAVT-45, potentially maintaining therapeutic abiraterone concentrations over 24 hours.
The key Phase III trial successfully achieved its primary goal of demonstrating therapeutic equivalence between TAVT-45 and ZYTIGA in patients with mCRPC and high-risk mCSPC, while also showing a similar safety profile. The NDA is currently being prepared. Tavanta is exploring strategic options for this easy-to-swallow alternative to ZYTIGA, aimed at patients with metastatic prostate cancer who have difficulty swallowing.
Candel Therapeutics’ CAN-2409
CAN-2409 (aglatimagene besadenovec) is an engineered gene construct that utilizes a replication-defective adenovirus to deliver the thymidine kinase gene from Herpes Simplex Virus. This treatment is administered via direct injection into the tumor or targeted tissue. This localized approach is thought to reduce the likelihood of developing anti-drug antibodies and the systemic side effects that can occur with systemic treatments. The adenoviral vector facilitates the delivery of the HSV-thymidine kinase gene into the tumor cells at the injection site. To date, CAN-2409 has shown a favorable safety profile, with over 950 patients treated across various solid tumor types.
In September 2021, CAN-2409 received Fast Track designation for treating localized, primary prostate cancer in combination with radiation therapy to enhance the local control rate. Candel is currently studying the effects of CAN-2409 in patients with nmPC.
In August 2024, Candel Therapeutics revealed that they anticipate topline disease-free survival results from the Phase III randomized controlled trial of CAN-2409 in patients with localized intermediate/high-risk prostate cancer, as well as topline progression-free survival results from the Phase IIb randomized controlled trial of CAN-2409 in the active surveillance cohort with localized low/intermediate-risk prostate cancer, both expected in the fourth quarter of 2024.
Explore more about Adenovirus-associated Oncolytic Virus Therapies in late-stage development
Lantheus’ 177Lu-PNT2002
177Lu-PNT2002 is a radioligand therapy candidate based on lutetium-177 that specifically targets the prostate-specific membrane antigen (PSMA). It integrates a PSMA-targeted ligand known as PSMA-I&T with the beta-emitting radioisotope, no-carrier-added lutetium-177. In December 2022, Lantheus obtained exclusive worldwide commercialization rights for 177Lu-PNT2002 from POINT, a subsidiary of Lilly, excluding specific Asian regions.
In April 2023, the FDA granted Fast Track designation to 177Lu-PNT2002 for treating mCRPC. Additionally, in September 2024, Lantheus presented new clinical data from the initial topline results of the SPLASH Phase III trial. This trial assessed the effectiveness of 177Lu-PNT2002, a PSMA-targeted radioligand therapy, given at a dose of 6.8 GBq every eight weeks for up to four cycles in mCRPC patients who had progressed on an androgen receptor pathway inhibitor (ARPI), during the European Society of Medical Oncology (ESMO) Congress 2024.
In December 2023, topline results from the pivotal Phase III SPLASH study revealed a 29% reduction in the likelihood of radiographic progression or death in patients with mCRPC who had progressed on an ARPI. However, overall survival (OS) data was still immature, with only 46% of the protocol-specified OS target events having occurred.
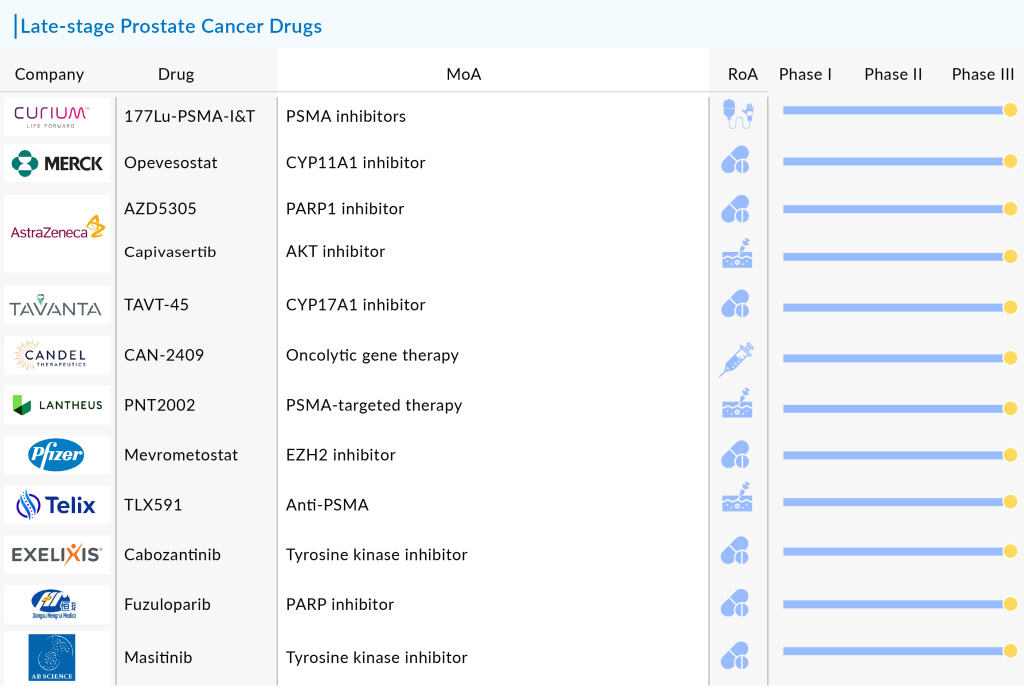
Pfizer’s Mevrometostat
Mevrometostat is a potent and selective small-molecule inhibitor of EZH2, effectively blocking the activity of the enhancer of zeste homolog 2 (EZH2) protein, which plays a role in the growth of prostate cancer cells. One of the proteins targeted by this mechanism is H3K27Me3. In Phase I/II study (part 2A), exploring various doses of Mevrometostat combined with enzalutamide and androgen deprivation therapy demonstrated a manageable safety profile and initial signs of efficacy, including a reduction in PSA levels by ≥50% from baseline (PSA50) and peripheral pharmacodynamic modulation in patients with CRPC.
In August 2024, Pfizer launched the first pivotal Phase III trial for prostate cancer drug mevrometostat in treating mCRPC. This medication may become a standard treatment option for patients in the future. Pfizer provided new Phase I data on the investigational EZH2 inhibitor mevrometostat used alongside XTANDI for the treatment of mCRPC. The registrational data for the combination of mevrometostat and XTANDI in patients with post-abiraterone mCRPC and treatment-naïve mCRPC is anticipated in the latter half of 2025 and later. Based on the company’s presentation in February 2024, mevrometostat is projected to be launched by 2026 for patients with post-abiraterone mCRPC.
Get an in-depth analysis of all the prostate cancer abstracts presented at the ESMO 2024
Telix Pharmaceuticals’ TLX591
TLX591 is the leading radiolabeled antibody-drug conjugate (rADC) from Telix, targeting PSMA. The ProstACT series of studies, which includes the Phase II/III ProstACT GLOBAL trial, is assessing the efficacy and safety of TLX591 in prostate cancer, spanning from initial recurrence to advanced metastatic stages. The company is also conducting clinical trials in Australia.
Additionally, TLX591 is undergoing further evaluation in the Phase III ProstACT GLOBAL trial for first and second-line treatment of mCRPC, with preparations underway to enroll patients at its initial US sites. This innovative trial design provides physicians with the option of using either androgen receptor inhibition or docetaxel chemotherapy, aligning with real-world care standards and demonstrating Telix’s ongoing commitment to innovation in prostate cancer treatment and improved patient outcomes.
AstraZeneca’s TRUQAP
Capivasertib is a new pyrrolopyrimidine derivative and an orally available inhibitor of the serine/threonine-protein kinase AKT that has potential antitumor effects. It binds to and inhibits all AKT isoforms, preventing the phosphorylation of AKT substrates involved in crucial cellular processes such as cell division, apoptosis, and the metabolism of glucose and fatty acids. Numerous solid tumors and blood cancers exhibit dysregulated PI3K/AKT/mTOR signaling due to mutations in various signaling components. By targeting AKT, a central element in the PI3K/AKT signaling pathway, this drug may be utilized as either monotherapy or in combination with other treatments for different types of human cancers.
The company has completed Phase Ib and Phase I/II trials of capivasertib for prostate cancer treatment. Additionally, AstraZeneca is currently exploring the use of capivasertib alongside ZYTIGA in Phase III CAPItello-281 study for mHSPC, as well as its clinical development in combination with docetaxel in the CAPItello-280 study for mCRPC.
According to the AstraZeneca H1 2024 clinical trial appendix report released in July 2024, the company expects to receive data from the Phase III CAPItello-281 trial (NCT04493853) for mHSPC in the second half of 2024, and from CAPItello-280 (NCT05348577) for mCRPC in 2025 and beyond.
Exelixis/Ipsen/Takeda’s CABOMETYX
Exelixis’ primary prostate cancer drug, cabozantinib, is a targeted therapy that blocks the function of various receptor tyrosine kinases, such as MET, AXL, VEGF receptors, and RET. These kinases play roles in normal cellular activities as well as in pathological processes like cancer development, metastasis, tumor blood vessel formation, and resistance to several treatments, including immune checkpoint inhibitors. Cabozantinib was discovered by Exelixis and the company holds exclusive rights to market it in the United States.
Exelixis is working with its partners on a global development strategy for cabozantinib, with Ipsen and Takeda handling the prostate cancer drug’s development in their respective regions. According to the findings from the Phase III CONTACT-02 pivotal study presented in September 2024, Exelixis plans to submit a supplemental New Drug Application (sNDA) to the US FDA for the use of cabozantinib in combination with atezolizumab for mCRPC later this year.
Jiangsu HengRui Medicine’s Fuzuloparib
Fuzuloparib, developed by Hengrui, is a new and effective orally active PARP inhibitor. Non-clinical studies indicated high bioavailability, and clinical data showed stable serum concentrations, minimal gastrointestinal side effects (≥ Grade 3 severity), and a low rate of treatment discontinuation due to adverse effects in patients using fuzuloparib.
When taken orally, fuzuloparib inhibits PARP 1 and 2, interfering with the base excision repair (BER) pathway responsible for DNA repair. This interference results in increased DNA strand breaks, greater genomic instability, and ultimately leads to apoptosis. Currently, fuzuloparib is undergoing a Phase III trial for mCRPC in combination with abiraterone, and a Phase II trial in combination with apatinib.
AB Science’s Masitinib
Masitinib is an orally taken tyrosine kinase inhibitor that targets mast cells and macrophages by blocking a specific set of kinases. Its distinctive mechanism allows for potential development in various oncology conditions, inflammatory diseases, and certain central nervous system disorders. Due to its immunotherapeutic effects in oncology, masitinib may influence survival rates, either on its own or when used alongside chemotherapy.
By acting on mast cells and microglia, masitinib inhibits the activation of inflammatory processes, which can alleviate symptoms related to specific inflammatory and central nervous system diseases, as well as slow their progression. Currently, masitinib is being explored for use as a first-line treatment for mCRPC.
According to a press release from March 2024, following the positive results of the initial Phase III study of masitinib combined with docetaxel, the company is preparing to submit plans for a confirmatory Phase III study in 2024. This follows guidance from the EMA and FDA, both of which advised that the focus should be on demonstrating benefits in radiographic progression-free survival rather than proving efficacy in overall survival.
The company holds two patents related to masitinib, covering its composition and synthesis process, with protection extending until 2028, including a potential patent term extension. In June 2023, AB Science announced that the European Patent Office had issued a Notice of Allowance for a patent concerning the treatment of mCRPC using its leading compound, masitinib, based on the results of study AB12003. This new European patent will provide intellectual property protection for masitinib in the treatment of mCRPC until 2042.
The Notice of Allowance indicates that the European Patent Office plans to grant the patent application, EP4175639A1, once specific formal procedural steps are completed. Once granted, the patent will remain in effect until May 2042. A European Notice of Allowance is issued after an examiner has determined that a patent application meets all the criteria for patentability under the European Patent Convention.
What’s Ahead in Prostate Cancer Drug Landscape?
The prostate cancer drug landscape is on the brink of a transformative era, marked by groundbreaking advancements and innovative therapies. As research continues to unveil the complexities of this disease, new drug candidates are emerging, offering hope to patients and families alike. With promising clinical trials and regulatory approvals on the horizon, patients can expect more effective treatment options that not only prolong life but also enhance its quality.
In addition to novel therapeutics, the integration of artificial intelligence and biomarker-driven approaches is revolutionizing how we approach prostate cancer treatment. These cutting-edge technologies are paving the way for earlier detection and tailored treatment plans that consider each patient’s unique genetic profile. As the landscape evolves, collaborations between pharmaceutical companies, academic institutions, and biotech firms will accelerate the development of breakthrough therapies, ensuring that prostate cancer patients receive the most advanced and effective care possible. The future is filled with promise, as we stand at the threshold of a new era in prostate cancer treatment that prioritizes innovation, patient-centricity, and improved outcomes.

FAQs
The US had 1.5 million diagnosed prevalent cases of prostate cancer in 2023, a number that is projected to rise by 2034 due to factors such as an aging population, improved screening techniques that enable earlier detection, lifestyle and environmental changes, and advancements in medical technology that enhance diagnosis and treatment options.
Currently approved prostate cancer drugs include XTANDI (Astellas/Pfizer), ZYTIGA (Janssen), ERLEADA (Janssen), NUBEQA (Bayer), LYNPARZA (AstraZeneca), RUBRACA (Pharma&Schwiez), TALZENNA (Pfizer), AKEEGA (Janssen), JEVTANA (Sanofi), and PLUVICTO (Novartis).
Key promising 12 prostate cancer drugs in clinical trials include Curium’s 177Lu-PSMA-I&T, Merck/Orion’s Opevesostat, AstraZeneca’s AZD5305, Tavanta Therapeutics’ TAVT-45, Candel Therapeutics’ CAN-2409, Lantheus’ 177Lu-PNT2002, Pfizer’s Mevrometostat, Telix Pharmaceuticals’ TLX591, AstraZeneca’s TRUQAP, Exelixis/Ipsen/Takeda’s CABOMETYX, Jiangsu HengRui Medicine’s Fuzuloparib, and AB Science’s Masitinib.
Key companies such as Eli Lilly and Company, Curium, Tavanta Therapeutics, Candel Therapeutics, Lantheus, Pfizer, Dongkook Pharmaceutical, Telix Pharmaceuticals, AstraZeneca, Exelixis, Jiangsu HengRui Medicine, SOTIO, AB Science, Bristol Myers Squibb, and Merck are currently the top runners with their respective candidates in the late stages of development.
Downloads
Article in PDF
Recent Articles
- Clover’s SCB-1019 RSV Revaccination Study Clears U.S. IND; FDA Approves AMVUTTRA for ATTR-CM CV R...
- Hologic complements Focal; Allogene for IPO; Gamida, Novartis-backed cell therapy startup; Aduro ...
- Phase III RUBY Trial of Jemperli Plus Chemotherapy Updates; FDA Approves Roche’s Vabysmo for RVO;...
- Takeda’s ADZYNMA Approved by FDA; AskBio Presents Preliminary Data from Phase I Trial of Gene The...
- Prostate Cancer Awareness Month: Early Detection Saves Lives!!



