6 Promising Late-stage NASH Drugs to Challenge REZDIFFRA’s Supremacy
Oct 14, 2024
Table of Contents
At present, REZDIFFRA stands as the only approved NASH drug to battle the devastating disease. This once-daily, oral THR-β agonist received a fast-track nod from the US FDA in March 2024, thanks to the impressive outcomes of the Phase III MAESTRO-NASH trial. But with one trailblazer already in the ring, the race is heating up—now every other contender is gunning to be the next in line, eager to claim the coveted title of the second approved NASH therapy. The game is on!
Want the full scoop on REZDIFFRA’s rise? Check out our blog @ REZDIFFRA’s Trailblazing Journey in NASH Treatment
Downloads
Article in PDF
Recent Articles
- Empowering Change: REZDIFFRA’s Trailblazing Journey in NASH Treatment
- Game-Changers in Non-Alcoholic Steatohepatitis (NASH) Treatment: Insights into Novel Drug Classes
- Gene-edited T cells for diabetes treatment; Pliant raised $144 million; Accent, AstraZeneca aim R...
- Algernon’s NP-120; FDA nod to 4DMedical tool; SaNOtize’s NORSTM trial; Pole’s c...
- Notizia
The Race for the Next Best NASH Drug
As the obesity and metabolic disorder crisis worsens, pharmaceutical companies are racing to develop the next game-changing NASH treatment. The competition to create the most effective therapy is heating up, with pharmaceutical giants leading clinical trials that could potentially reshape the NASH treatment landscape. Some of the NASH drugs in the pipeline that can be launched in the coming years include Saroglitazar Magnesium (Zydus Therapeutics), Obeticholic Acid (OCA) (Intercept Pharmaceuticals), Efruxifermin (EFX) (Akero Therapeutics), Pegozafermin (89bio), MSDC-0602K (Cirius Therapeutics), Lanifibranor (Inventiva Pharma), and Semaglutide (Novo Nordisk A/S), and others.
Let’s dive into these promising NASH drugs in development and how these breakthrough therapies are expected to change the future of care for millions living with this silent liver disease.
Galmed Research and Development, Ltd.’s Aramchol
Current Phase – III
Aramchol, a novel synthetic molecule developed by Galmed Pharmaceuticals Ltd., is emerging as a promising candidate in the fight against non-alcoholic steatohepatitis, particularly in patients with fibrosis. The drug is currently in Phase III of clinical development, and the one-year results from the Open-Label phase of the global ARMOR trial, published on September 25, 2024, provide important insights into its efficacy. Notably, treatment with Aramchol 300mg BID led to a significant improvement in histological fibrosis for 39% of patients, with pathology assessments revealing a remarkable 61% showing improvement after at least 48 weeks of treatment. These findings suggest that Aramchol’s mechanism of action—specifically, its ability to inhibit SCD-1 and enhance fatty acid oxidation—may play a crucial role in mitigating liver fibrosis and addressing the underlying metabolic dysfunction associated with NASH.
Furthermore, the trial utilized an AI-assisted digital pathology approach, which underscored Aramchol’s potential by demonstrating that all treated patients met the criteria for fibrosis improvement based on pre-specified reductions in the Fibrosis Composite Severity score. This level of efficacy, coupled with the ongoing exploration of SCD-1 inhibition in various indications, highlights Aramchol’s position as a leading therapeutic candidate in the NASH clinical landscape. As Galmed continues to gather data, the momentum surrounding Aramchol could significantly impact the treatment paradigm for NASH, paving the way for more effective management of this complex disease.
Akero Therapeutics’ Efruxifermin (EFX)
Current Phase – III
Efruxifermin (EFX) is Akero’s lead product candidate for the treatment of MASH. EFX is a differentiated Fc-FGF21 fusion protein, engineered to mimic the balanced biological activity of native FGF21, an endogenous hormone that helps alleviate cellular stress and regulates metabolism throughout the body. EFX is designed for convenient, once-weekly subcutaneous dosing. With its consistent and significant observed effects, EFX has the potential to become a best-in-class treatment option for MASH, pending approval.
In June 2024, Akero initiated the SYNCHRONY Outcomes study, a Phase III trial that aims to evaluate the efficacy and safety of Efruxiferminin patients with compensated cirrhosis (F4 stage) due to MASH. This trial marks a significant step forward in advancing EFX toward potential regulatory approval.
89bio’s Pegozafermin
Current Phase- III
Pegozafermin (BIO89-100) is an innovative therapeutic compound that specifically targets and activates the fibroblast growth factor 21 (FGF21) receptor. FGF21 is a crucial hormone involved in the regulation of glucose and lipid metabolism, and it has emerged as a key player in addressing metabolic disorders, particularly in the context of MASH. By modulating the activity of this hormone, Pegozafermin has shown potential therapeutic benefits in improving metabolic parameters and reducing liver fat accumulation, which are critical factors in the management of MASH.
Early-stage NASH clinical trials and preclinical studies of BIO89-100 have demonstrated promising results, highlighting its ability to enhance metabolic health in patients suffering from MASH. In May 2024, 89bio took a significant step forward by initiating the Phase III ENLIGHTEN-Cirrhosis Trial. This trial focuses on evaluating the safety and efficacy of Pegozafermin in patients with compensated cirrhosis due to MASH, aiming to establish its potential as a viable treatment option for this challenging condition. The outcomes of this trial could play a pivotal role in shaping the future of MASH therapy.
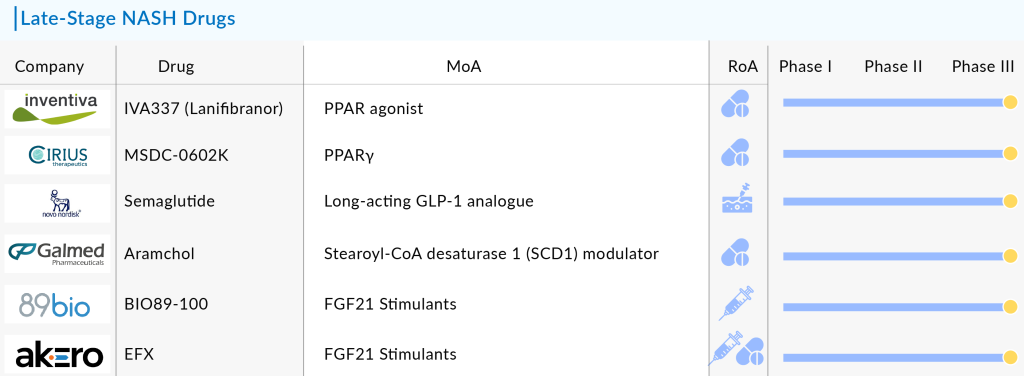
Cirius Therapeutics’ MSDC-0602K
Current Phase – III
MSDC-0602K, a second-generation oral insulin sensitizer, is making waves in the NASH treatment landscape. This innovative drug is designed to selectively target the mitochondrial pyruvate carrier (MPC), which plays a pivotal role in managing the cellular effects of overnutrition—a major contributor to NAFLD, NASH, and Type 2 diabetes. Unlike other treatments, MSDC-0602K minimizes direct PPAR-gamma activation, allowing it to offer a more targeted approach. In preclinical studies, modulating the MPC has shown promising results, improving insulin sensitivity, lipid metabolism, and reducing inflammation—key factors in the progression of NASH. Now in Phase III clinical trials, MSDC-0602K stands as a potential game-changer in the NASH therapy market, offering hope to those battling this complex metabolic disease. If successful, this drug could mark a significant advancement in how NASH is treated, addressing not only liver health but also the broader metabolic disruptions at the heart of the condition.
In a recent update, Cirius Therapeutics, the developer of MSDC-0602K, presented new data on June 5, 2024, at the European Association for the Study of Liver Disease (EASL) annual meeting. The company, in collaboration with Dr. Kyle McCommis’ lab at St. Louis University, highlighted the unique potential of combining MSDC-0602K with GLP-1 receptor agonists (GLP-1s). This combination may help address body composition issues linked to GLP-1 therapies, which, while beneficial for metabolic health, often come with side effects related to fat redistribution and muscle mass loss. The data points to azemiglitazone (MSDC-0602K) as a promising option to mitigate these challenges while enhancing the therapeutic efficacy of GLP-1s—offering a more holistic approach to tackling NASH and its associated metabolic disorders.
Inventiva Pharma’s Lanifibranor
Current Phase: Phase III
Lanifibranor, Inventiva’s leading NASH drug candidate, is an orally available small molecule designed to induce antifibrotic, anti-inflammatory, and beneficial vascular and metabolic effects. It works by activating all three isoforms of peroxisome proliferator-activated receptors (PPAR)—nuclear receptor proteins that regulate gene expression. Unlike other PPAR agonists that target only one or two isoforms, lanifibranor provides a balanced activation of PPARα and PPARδ, along with partial activation of PPARγ. The FDA has recognized its potential by granting Breakthrough Therapy in August 2021 and Fast Track designation in September 2021 for the treatment of NASH. Lanifibranor is currently in Phase III clinical trials for NASH.
Novo Nordisk A/S’s Semaglutide
Current Phase: Phase III
Semaglutide is a GLP-1 receptor agonist initially developed for managing type 2 diabetes and aiding weight loss. By mimicking the natural hormone GLP-1, semaglutide enhances insulin secretion, suppresses glucagon release, and slows gastric emptying, contributing to improved glycemic control and weight reduction. Its efficacy in metabolic improvement has prompted exploration for treating NASH, a condition linked to obesity and insulin resistance.
On March 18, 2021, Gilead Sciences, Inc. and Novo Nordisk A/S expanded their clinical collaboration in NASH, initiating a Phase IIb double-blind, placebo-controlled study to evaluate the safety and efficacy of semaglutide alongside Gilead’s investigational FXR agonist cilofexor and ACC inhibitor firsocostat. This trial will enroll approximately 440 patients with compensated cirrhosis (F4) due to NASH, focusing on liver fibrosis improvement and NASH resolution. This study follows a Phase IIa proof-of-concept trial in 2020, which showed that semaglutide combined with cilofexor and/or firsocostat was well tolerated, with significant improvements in liver health biomarkers but no statistically significant differences in liver stiffness or fibrosis scores among treatment groups.
Currently, a Phase III trial, ESSENCE, is underway to investigate the use of semaglutide for NASH-related fibrosis, marking a significant step forward in the quest for effective NASH treatment. Although recent Phase II trials for NASH-related cirrhosis did not demonstrate significant improvements in fibrosis or NASH resolution, the trials did show notable cardiometabolic benefits. This suggests that while semaglutide may not directly resolve liver fibrosis at this stage, its broader metabolic effects could still play a crucial role in managing NASH’s associated conditions. The ongoing Phase III trial holds promise, as researchers fine-tune the treatment to tackle both liver damage and metabolic disturbances, pushing the boundaries of what’s possible in NASH therapy.
Explore the game-changers in the NASH pipeline in our blog @ Insights into Novel Drug Classes
Conclusion
As we stand at the crossroads of innovation and necessity, the future of NASH treatment is glowing with promise. With only one approved NASH drug currently on the market, the urgent need for effective therapies has never been more evident. Emerging NASH drugs like Saroglitazar, Obeticholic Acid, Efruxifermin, and others are not just potential therapies; they represent beacons of hope for millions grappling with this elusive condition. These cutting-edge NASH therapies, backed by robust clinical trials, are poised to reshape the landscape of liver health and offer patients renewed optimism in managing their disease.
Curious about NASH and its impact? Learn more @ Nonalcoholic Steatohepatitis (NASH) Landscape
Imagine a world where NASH is no longer a silent, progressive burden but a manageable condition, thanks to innovative NASH drugs that target the root causes of liver damage and inflammation. As pharmaceutical giants continue investing in research and development, we are inching closer to a breakthrough moment in NASH care. With these promising therapies on the horizon, patients and healthcare professionals alike can look forward to a new era of personalized NASH treatment options that not only alleviate symptoms but tackle the underlying metabolic disturbances at play. The journey is just beginning, and the potential to transform lives through advanced therapies is an exciting horizon worth pursuing.

Downloads
Article in PDF
Recent Articles
- Notizia
- A Beacon of Hope: A Look at the Latest Advances in Emerging Therapies for Liver Cirrhosis Treatment
- Bristol Myers Squibb’ Karuna Therapeutics Buyout; Orchard Therapeutics’ Lenmeldy FDA Approval; Ma...
- Rapt Therapeutics files IPO; Novartis stakes $80M for NASH
- Algernon’s NP-120; FDA nod to 4DMedical tool; SaNOtize’s NORSTM trial; Pole’s c...