CDK7 Inhibitors: A Promising Class in Cancer Therapeutics
Nov 04, 2024
Table of Contents
Cyclin-dependent kinase 7 (CDK7) belongs to the CDK family of serine/threonine protein kinases and plays a crucial role in regulating the cell cycle and mRNA transcription. It is often overexpressed in various cancers, making it a promising target for cancer treatment due to its involvement in both transcription and cell cycle regulation.
Additionally, CDK7 can directly influence the activities of the estrogen receptor, a key factor in breast cancer progression. While several CDK4/6 inhibitors are approved for use alongside endocrine therapies in treating ER+ breast cancer, resistance to these drugs is becoming a significant issue. Notably, breast cancer cells that have developed resistance to palbociclib still respond to THZ1, indicating that CDK7 inhibitors could be beneficial after resistance to other CDK-targeting drugs develops.
Downloads
Article in PDF
Recent Articles
- 5 Novel CDK7 Inhibitors Pushing the Boundaries in Oncology
- Byondis’s HER2-targeting ADC trastuzumab duocarmazine; AbbVie Migraine Drug Atogepant; Grünenthal...
- Intellia’s Quest with CRISPR; Innovent, Synaffix ADC Tech Deal; Lilly’s Diabetes Bloc...
- GSK consolidates DT and TT vaccine, AZ gears up for FDA filing, Novartis comes up with flex prici...
- Lassen’s anti-IL-11 antibody; PTC’s COVID-19 trial; Lenzilumab’s result; Orca raises $192M
The Role of CDK7 in Cancer
CDK7, a member of the cyclin-dependent kinase family, plays a pivotal role in regulating both the cell cycle and gene transcription, making it a critical player in cancer biology. As part of the CDK-activating kinase (CAK) complex, CDK7 is responsible for activating other cyclin-dependent kinases, such as CDK1, CDK2, CDK4, and CDK6, which drive various phases of the cell cycle. In many cancers, the dysregulation of these CDKs leads to unchecked cellular proliferation, contributing to tumorigenesis. Furthermore, CDK7’s involvement in the G1-S and G2-M transitions of the cell cycle underscores its importance in maintaining proper cell division and genomic integrity, processes that are often compromised in cancerous cells.
In addition to its role in cell cycle regulation, CDK7 is crucial for transcriptional control, particularly through its phosphorylation of RNA polymerase II. This phosphorylation activates RNA polymerase II, allowing it to initiate transcription and process pre-mRNA, which is essential for the expression of genes that promote cell growth and survival. In many tumors, including those driven by oncogenes like MYC and E2F, CDK7-mediated transcriptional activation is often hyperactivated. This overexpression not only drives cancer cell proliferation but also supports the maintenance of aggressive phenotypes, highlighting CDK7 as a key factor in tumor progression and resistance to conventional therapies.
Moreover, the dual function of CDK7 in both cell cycle and transcriptional regulation creates a unique therapeutic opportunity. Targeting CDK7 with specific inhibitors can potentially disrupt these pathways simultaneously, leading to the arrest of cancer cell proliferation and induction of apoptosis. This dual mechanism is particularly relevant in cancers with high transcriptional demands, where CDK7 inhibition can effectively starve cancer cells of the necessary signals for growth. The emerging understanding of CDK7’s roles in cancer has spurred significant interest in developing CDK7 inhibitors as a novel class of therapeutics, aimed at addressing the challenges posed by traditional cancer treatments.
Target Patient Pool of CDK7 Inhibitors
Breast cancer incidence and death rates increase with age until the seventh decade. The decrease in incidence rates that occur in women 80 years of age and older may reflect lower rates of screening, the detection of cancers by mammography before 80 years of age, and/or incomplete detection.
According to the DelveInsight estimates, the total incident population of HR+/HER2- breast cancer in the 7MM was nearly 476K cases in 2023. The cases in the 7MM are expected to increase during the forecast period, i.e., 2024–2034.
Approximately 85% of patients diagnosed with metastatic breast cancer have had an early-stage breast cancer diagnosis. However, most patients with early-stage breast cancer do not go on to develop metastatic disease.
CDK7 as a Therapeutic Target
Interest in targeting CDK7 as a potential therapy has persisted for over a decade, with notable advancements made in recent years. The CDK7 inhibitor field is rapidly expanding, featuring various chemical classes and promising selectivity for kinases. These compounds utilize diverse mechanisms of inhibition, including conventional competition, irreversible binding, and specific CDK7 degradation through heterobifunctional agents.
Comparative analyses show that CDK7 expression is significantly elevated in tumor tissues across a range of cancers, including breast cancer. Targeting CDK7 has demonstrated strong effects on cancer cell proliferation, migration, and drug resistance in several malignancies, such as breast, lung, hepatocellular, thyroid, glioblastoma, gastric, pancreatic, gallbladder, colorectal cancers, osteosarcoma, lymphomas, and leukemia. CDK7 holds substantial promise as a novel therapeutic agent for various conditions, including castration-resistant prostate cancer, HR+ HER2− metastatic breast cancer, triple-negative breast cancer, and colorectal cancer.
Preclinical and clinical data particularly highlight the potential of targeting CDK7 in advanced HR+/HER2− breast cancer cases that have progressed following treatment with a CDK4/6 inhibitor alongside hormonal therapy.
In studies assessing CDK7 levels in normal breast epithelium and a set of 12 breast cancer cell lines, immunoblotting showed increased CDK7 protein expression in cancerous tissues compared to normal breast epithelium. This finding was supported by a thorough analysis of CDK7 mRNA levels in breast cancer patients using multiple public databases, including METABRIC microarray, Oncomine, GEPIA2, Human Protein Atlas (HPA), and the GENT database. The results indicated significantly higher CDK7 protein levels in HR-positive and HER2-positive breast cancer subtypes compared to TNBC.
Although the CDK7 inhibitor drug has not yet received approval from the US FDA for use, researchers are enhancing the technique through clinical trials to improve its effectiveness. Several key companies such as Carrick Therapeutics (Samuraciclib), Qurient Therapeutics (Q901), Syros Pharmaceuticals (SY-5609), TYK Medicine (TY-2699a), and GT Apeiron/Exscientia (GTAEXS617) are currently assessing CDK7 inhibitor drugs in clinical trials.
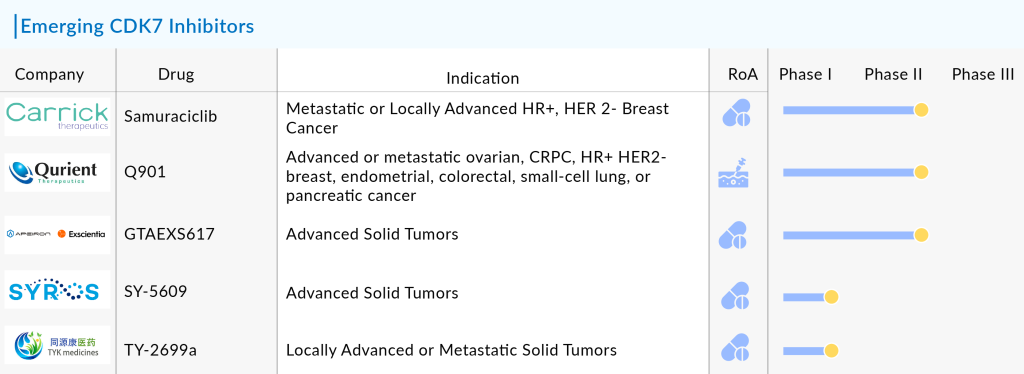
The anticipated launch of these emerging therapies are poised to transform the CDK 7 inhibitors market landscape in the coming years. As these cutting-edge therapies continue to mature and gain regulatory approval, they are expected to reshape the CDK 7 inhibitors market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
CDK7 Inhibitors: Challenges and Future Directions
Despite the promising therapeutic potential of CDK7 inhibitors in oncology, several challenges hinder their clinical application. One of the primary concerns is the potential for toxicity, as CDK7 is involved in essential cellular processes beyond tumorigenesis, including normal transcriptional regulation in healthy cells.
This can lead to adverse effects, particularly with prolonged use. Developing selective CDK7 inhibitors that can differentiate between cancerous and normal cells is crucial to minimizing toxicity while maximizing efficacy. Additionally, understanding the pharmacokinetics and optimal dosing regimens for these inhibitors will be essential to achieving the desired therapeutic outcomes without overwhelming the patient’s system.
Another significant challenge is the development of resistance mechanisms by tumors. As with many targeted therapies, cancer cells may adapt over time, leading to diminished sensitivity to CDK7 inhibitors. Identifying the biomarkers associated with responsiveness and resistance is vital for refining treatment protocols.
Ongoing research aims to elucidate the pathways involved in resistance and to develop combination therapies that can counteract these mechanisms. For instance, combining CDK7 inhibitors with other targeted therapies, such as CDK4/6 inhibitors or PARP inhibitors, may enhance their effectiveness and reduce the likelihood of resistance.
Looking to the future, the landscape for CDK7 inhibitors appears promising, especially with advancements in biomarker-driven therapies. Identifying specific cancer types or genetic profiles that are more likely to respond to CDK7 inhibition can enable personalized treatment approaches.
Pharmaceutical companies and researchers are searching for the next generation of CDK inhibitors to use either alone or alongside current therapies, aiming to address the issue of acquired resistance to CDK4/6 inhibition. Meanwhile, the progress of CDK7 inhibitors is accelerating, with several drugs now in clinical development.
Gaining a more thorough understanding of CDK7 biology will ultimately help identify specific genes that are selectively reliant on CDK7. This knowledge could lead to more effective selection of particular cancer types and enhance personalized treatment strategies.
Additionally, leveraging combination strategies could improve patient outcomes, particularly in complex tumor environments where multiple signaling pathways are activated. Continued clinical trials and research will be essential to fully understand the potential of CDK7 inhibitors, optimize their use, and integrate them into existing treatment paradigms for various malignancies, ultimately enhancing the arsenal against cancer.

Downloads
Article in PDF
Recent Articles
- Edwards’ Sapien 3 with Alterra Prestent; Koios Medical’s breast, thyroid cancer-spotting AI; Line...
- GSK consolidates DT and TT vaccine, AZ gears up for FDA filing, Novartis comes up with flex prici...
- AstraZeneca’s Voydeya FDA Approval; Akebia’s Vafseo FDA Approval; Bristol Myers Squibb’s Phase II...
- Ypsomed-CamDiab’s Collaboration; Vela Diagnostics’s NGS-Based Pan-Cancer Panels; Fresenius&...
- Eli Lilly’s Jaypirca Approval; Novartis’ Adakveo EMA Review; Janssen’s CARTITUDE-4 Study of CARVY...