CAR-T Cells vs. CAR-Exosome Agents: Exploring the Future of Cancer Immunotherapy
Nov 15, 2024
Table of Contents
Immunotherapy has revolutionized the treatment landscape for cancer, offering hope for patients who have exhausted traditional therapeutic options. In the ever-evolving field of immunotherapy, two emerging approaches—CAR-T cells and CAR-Exosome agents—are garnering attention for their potential to revolutionize the treatment of cancer.
Both harness the power of the body’s immune system to target and destroy cancer cells, but they differ in their mechanisms of action, manufacturing processes, and risk profiles. Understanding the distinction between CAR-T cells and CAR-exosome agents is crucial for evaluating their potential to reshape cancer treatment.
Downloads
Article in PDF
Recent Articles
- Bristol Meyers Squibb & AbbVie partner; BioLineRx’s collaboration with MD Anderson Cancer Ce...
- Is Gene Therapy the Next Cancer Treatment Revolution?
- Immuno-Oncology (I-O) Therapeutics: The Key to Future Cancer Treatment
- A promising immune-stimulating target
- AKT Inhibitors: A potential Cancer Immunotherapy Target
What are CAR-T Cells?
CAR-T cell therapy involves genetically modifying a patient’s T-cells to express a receptor specific to a tumor antigen. These engineered T cells are then infused back into the patient’s body, where they seek out and destroy cancer cells expressing the target antigen.
The process begins with the isolation of T cells from the patient’s blood. These T cells are then engineered in the lab to express a chimeric antigen receptor (CAR), which consists of an extracellular domain that recognizes a specific tumor antigen, a transmembrane domain, and an intracellular signaling domain that activates the T cell upon antigen recognition. Once infused, the CAR-T cells activate and proliferate in response to the target tumor cells, leading to their destruction.
While CAR-T therapy has been revolutionary in the treatment of certain cancers, particularly in hematological malignancies, it does have limitations. One major challenge is cytokine release syndrome (CRS), an inflammatory response triggered by the activation of CAR-T cells, which can lead to severe side effects. Additionally, CAR-T cells are often associated with neurotoxicity and tumor antigen escape, where cancer cells evolve to evade the immune response. Furthermore, the time-consuming and costly process of manufacturing patient-specific CAR-T cells limits widespread application.
Enter CAR-Exosome Therapy
Exosomes are small, naturally occurring extracellular vesicles that are secreted by most cell types. They play a critical role in intercellular communication by carrying proteins, lipids, and genetic material, such as RNA, between cells. This ability to transfer molecular signals makes exosomes an attractive vehicle for therapeutic delivery.
Discover more about exosomes in “Exosomes: Tiny Messengers with Big Potential in Medical Science”
CAR-exosome therapy involves the modification of exosomes to express chimeric antigen receptors. These engineered exosomes, which can be derived from various cell types such as dendritic cells, mesenchymal stem cells, or even tumor cells, are designed to target specific tumor-associated antigens. Once these exosomes are engineered with the desired CAR, they can be infused into patients to deliver targeted therapies like CAR-T cells, but with distinct advantages.
Potential Benefits of CAR-exosome Therapy Over Traditional CAR-T Cell Therapy
In recent years, cancer immunotherapy has experienced groundbreaking advancements, particularly in the development of CAR-T therapy. This approach has shown promising results in treating various hematologic cancers, including leukemia and lymphoma. However, as with any emerging treatment, limitations such as toxicity, short-lived responses, and complex manufacturing processes have prompted researchers to explore alternative strategies. One such strategy is the use of CAR-modified exosomes, an innovative approach that could overcome some of the challenges associated with CAR-T therapy.
Reduced Toxicity
One of the most significant concerns with CAR-T cell therapy is the risk of cytokine release syndrome (CRS), a systemic inflammatory response triggered by CAR-T cells after they engage tumor cells. CRS can lead to severe complications, including organ failure. Additionally, CAR-T cells can cause neurotoxicity, leading to symptoms such as confusion, seizures, or encephalopathy. Because exosomes are non-living particles, they do not provoke the same level of immune activation as CAR-T cells, potentially reducing the incidence of CRS and neurotoxicity. The smaller size and natural lipid membrane of exosomes make them less immunogenic, lowering the likelihood of causing harmful systemic reactions.
More Stable and Easier to Manufacture
Exosomes are more stable than living cells and do not require the same degree of customization that CAR-T cells do. CAR-T therapy involves extracting T cells from the patient, genetically modifying them, expanding them in culture, and reinfusing them into the patient. This process can take weeks and is costly. In contrast, exosomes can be produced from healthy donor cells in a more standardized, scalable fashion. These donor-derived exosomes can be engineered with CARs targeting specific antigens, and then stored for later use, streamlining the manufacturing process. This could lower the cost, make the treatment more accessible, and shorten the treatment timeline.
Targeted Delivery of Multiple Cargoes
Exosomes are incredibly versatile delivery vehicles. Not only can they be engineered to display chimeric antigen receptors (CARs) on their surface, but they can also carry a variety of therapeutic cargo inside. This includes proteins, RNA (such as RNA interference agents or mRNA therapies), small molecules, or even other immune-modulating agents. By delivering a range of therapeutic agents, CAR-exosomes could address multiple aspects of cancer treatment simultaneously, including enhancing immune responses, inhibiting tumor growth, or even reprogramming the tumor microenvironment to become less immune-suppressive. This capability offers an edge over CAR-T cells, which are typically limited to a single therapeutic modality—activation of T cell immunity.
Enhanced Tumor Penetration
Exosomes are naturally adept at crossing biological barriers, including the blood-brain barrier (BBB), which is a significant obstacle for many conventional cancer therapies. Tumors often reside in difficult-to-reach anatomical locations, and CAR-T cells may have limited ability to effectively penetrate solid tumors. Exosomes, on the other hand, have a small size and a natural ability to enter various tissues more efficiently than larger immune cells like T cells. Their ability to navigate complex tumor microenvironments could make CAR-exosome therapy an attractive option for targeting not only blood cancers but also solid tumors, where CAR-T therapy has faced challenges.
Minimized Risk of On-Target, Off-Tumor Toxicity
One of the risks associated with CAR-T cells is on-target, off-tumor toxicity, which occurs when CAR-T cells mistakenly attack healthy tissues that express low levels of the target antigen. Since exosomes are smaller and lack the full immune response capabilities of T cells, the risk of such toxicity could be reduced. Exosomes can be designed to specifically target tumor cells by functionalizing their surface with specific ligands or CARs without inducing the full-blown immune activation that CAR-T cells do. This selectivity helps to minimize collateral damage to healthy tissues.
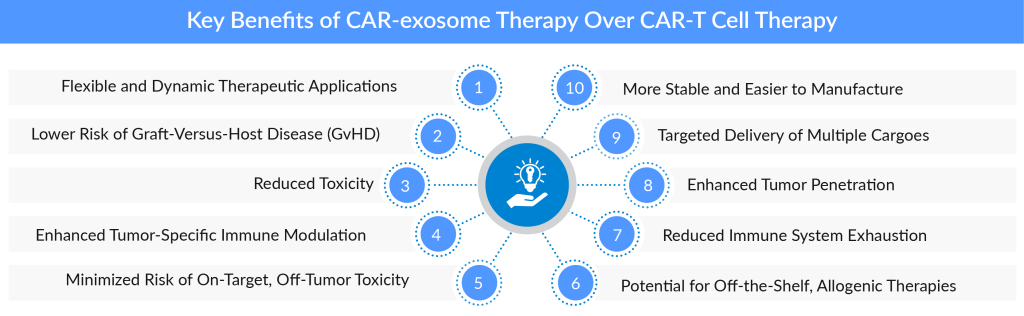
Reduced Immune System Exhaustion
The long-term persistence of CAR-T cells in the body can sometimes lead to immune exhaustion. After repeated activation, T cells may lose their effectiveness and become less responsive to further challenges. In contrast, CAR-exosomes are transient and do not persist in the body for long periods, which may reduce the likelihood of immune exhaustion. Their short lifespan could be seen as an advantage, as it minimizes prolonged immune activation and associated side effects.
Potential for Off-the-Shelf, Allogenic Therapies
Unlike CAR-T cells, which must be specifically derived from each patient, CAR-exosomes can be generated from healthy donor cells, allowing for off-the-shelf therapies. This could drastically reduce treatment costs and wait times for patients, enabling faster access to therapy. The ability to use allogenic (donor-derived) exosomes opens up the possibility for mass production and distribution, much like a traditional pharmaceutical product, making it easier to scale up production and administer treatments at a population level.
Enhanced Tumor-Specific Immune Modulation
CAR-exosomes have the potential to carry and deliver immune-modulating agents such as cytokines or small molecules that could directly alter the tumor microenvironment. For example, CAR-exosomes could deliver immune checkpoint inhibitors to tumors, turning the immune-suppressive environment into one that is more permissive to immune cell activity. This ability to deliver multiple immune modulators in tandem could potentially boost the overall effectiveness of the immune response against cancer cells.
Lower Risk of Graft-Versus-Host Disease (GvHD)
GvHD is a potential complication in allogeneic stem cell transplants, where the donor’s immune cells attack the recipient’s tissues. Since exosomes lack the full immune effector functions of T cells, they are less likely to induce GvHD, making CAR-exosome therapy a safer option in allogenic (donor-derived) treatments.
Flexible and Dynamic Therapeutic Applications
The CAR-exosome platform is highly versatile and can be adapted to various types of cancer. Exosomes can be engineered to carry different therapeutic cargo depending on the cancer type, the patient’s immune profile, or specific genetic mutations. This flexibility allows for the development of personalized, adaptive treatment strategies, optimizing the therapeutic potential for different cancers and patient populations.
Challenges and Future Outlook
Despite the promising advantages of CAR-exosome agents, there are still challenges to overcome. The process of engineering exosomes, ensuring consistent targeting, and controlling the release of therapeutic cargo requires further refinement. Additionally, the ability of CAR-exosomes to achieve the same level of tumor cell targeting and immune activation as CAR-T cells is still being investigated.
On the other hand, CAR-T cell therapy continues to evolve with strategies being developed to reduce toxicity, improve tumor targeting, and extend the duration of action. Advances in gene editing, such as CRISPR, and the development of off-the-shelf CAR-T therapies are likely to overcome some of the limitations faced by current CAR-T treatments.
In the near future, a combination of CAR-T and CAR-exosome therapies could provide a more comprehensive approach to cancer treatment, with each modality complementing the other. By leveraging the strengths of both, we could see a new era of precision immunotherapy that addresses the weaknesses of current therapies.

Downloads
Article in PDF
Recent Articles
- CAR-T Cell Therapy aka Miraculous Technology: Mapping the Market, Approved Therapies, Competitive...
- AKT Inhibitors: A potential Cancer Immunotherapy Target
- Chimeric Antigen Receptor T-cell Therapy Landscape
- Infusion Therapy Devices: Revolutionizing Healthcare Delivery
- CAR T-Cell Therapies in Non-Hodgkin’s Lymphoma Treatment: A Revolutionary Approach