Celiac Disease Treatment: Who Will Secure First-Mover Advantage?
Dec 02, 2024
Table of Contents
Celiac disease is considered a serious autoimmune disease, which is estimated to affect 1 in 100 people worldwide. Moreover, 2.5 million Americans are undiagnosed and are at risk for long-term health complications.
As per secondary, commonly known clinical types of celiac disease include Classical, Non-classical, and Sub-clinical. In the United States, the number of non-classical celiac disease was maximum compared to the other two types.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Top 6 Celiac Disease Therapies Progressing in Mid-Stage Trials
- Evaluating the Major Developments in the Smart Pills Market
- Celiac Disease: The Hidden Epidemic of Gluten Sensitivity
- Celiac Disease (CD) – Upcoming therapies to break the stagnancy
- Which Pharma Companies are Working to Mitigate the Burden of Digestive Disorders?
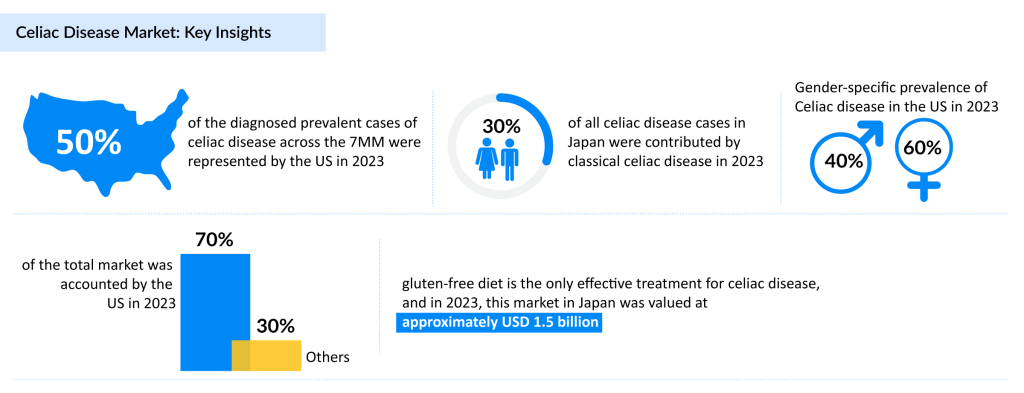
In 2023, the United States represented nearly 50% of the diagnosed prevalent cases of celiac disease across the 7MM, as per DelveInsight’s latest Celiac Disease Epidemiology Forecast Report. Celiac disease shows a notable gender disparity, with females being disproportionately affected.
For instance, in Germany, approximately 60% of diagnosed cases are female. In 2023, classical celiac disease comprised about 30% of all celiac disease cases in Japan. It is most commonly diagnosed in individuals aged 19-39, emphasizing the need for timely detection and management.
Current Approach in Celiac Disease Management
There are currently no FDA-approved drugs for celiac disease treatment. The only treatment currently available for celiac disease is a strict, lifelong gluten-free diet, which can be both expensive and difficult to maintain. Many patients struggle with incomplete intestinal healing and ongoing symptoms, often due to challenges in adhering perfectly to the diet. Even with their best efforts, accidental gluten ingestion through cross-contamination remains a significant risk, impacting their health and quality of life.
A survey of 1,255 Americans medically diagnosed with celiac disease revealed that, although 93% of respondents avoid gluten intentionally, nearly 73% still experience accidental gluten exposure annually, leading to symptoms. Around 36% reported unintentionally consuming gluten 1-5 times a month, and over 66% experienced severe or very severe symptoms. The most common symptoms were gastrointestinal (84%), neurological (56%), and psychological (40%).
Glucocorticoids are powerful anti-inflammatory agents that inhibit B cells and suppress cytokine production by regulatory T cells. Corticosteroids have been evaluated as a treatment for non-responsive celiac disease (NRCD) in cases where a gluten-free diet is ineffective, particularly for refractory celiac disease (RCD). However, their use is limited by systemic side effects.
For RCD types I (RCDI) and II (RCDII), pharmacological treatments improve symptoms and histology in only 30–40% of cases. While RCDI often responds to treatment, RCDII is typically less responsive to available therapies. Treatments for both types include budesonide, systemic corticosteroids, 6-mercaptopurine, cladribine, and mesalamine. Additional options for RCDI include mycophenolate mofetil and methotrexate. However, current treatments for RCDII do not appear to prevent progression to enteropathy-associated T-cell lymphoma (EATL), and mortality rates remain high despite aggressive interventions.
Recent progress in understanding the underlying mechanisms of celiac disease and identifying potential therapeutic targets has paved the way for exploring disease-specific pharmaceutical treatments. The FDA has recently emphasized the unmet needs of adult patients who, despite adhering to a gluten-free diet, continue to experience symptoms linked to celiac disease and exhibit persistent duodenal villous atrophy.
Promising celiac disease drug therapies are under development to ease the burden of managing celiac disease and enhance long-term health outcomes. Additionally, patients may be advised to take certain vitamin and mineral supplements, particularly during the first six months following diagnosis.
Emerging Celiac Disease Therapies: Who Will Be the Game-Changer?
Emerging therapies for celiac disease are focusing on novel approaches to manage or potentially cure the condition, which currently has no pharmacological treatments apart from a strict gluten-free diet. One promising avenue is the development of enzyme-based therapies. These enzymes, such as ALV003, are designed to break down gluten peptides in the digestive tract before they can trigger an immune response. By degrading gluten at the molecular level, these therapies aim to reduce inflammation and prevent damage to the small intestine, offering a potential therapeutic option for celiac patients who inadvertently consume gluten.
Another exciting area of research is the use of immunomodulatory agents that target the immune response in celiac disease. These treatments, like latiglutenase and Duo-001, aim to regulate the immune system’s exaggerated response to gluten. While still in clinical trials, these therapies could offer significant improvements in quality of life by reducing symptoms and enabling a more flexible diet. Additionally, there is interest in gluten immunotherapy, which attempts to desensitize the immune system to gluten through controlled exposure or by blocking specific immune receptors, offering a potential long-term solution for managing the disease beyond dietary restrictions.
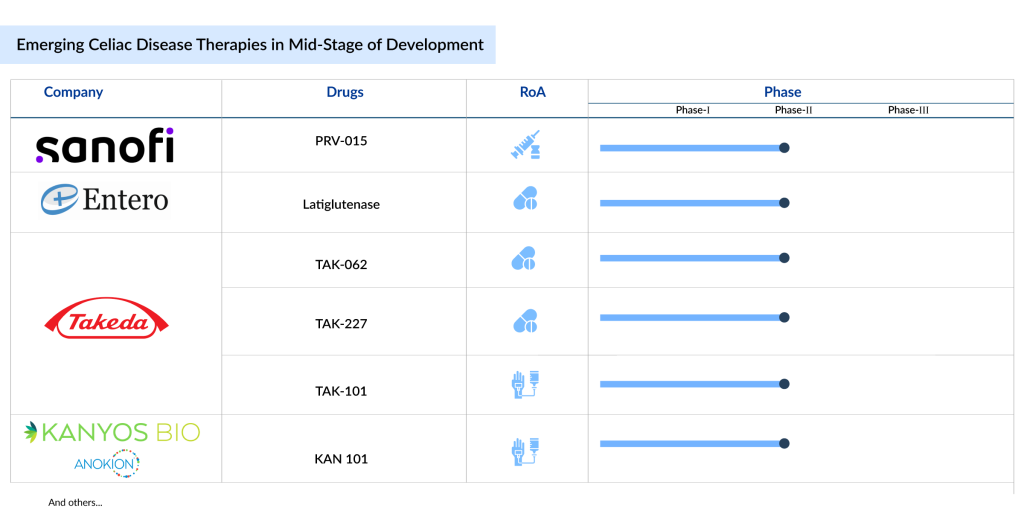
Currently, 25+ companies are active in the celiac disease treatment space across the globe, as per DelveInsight’s Celiac Disease Pipeline Report. Some of the promising drugs in the celiac disease pipeline include Latiglutenase (First Wave BioPharma), TAK-101; TAK-227; Zamaglutenase (Takeda), PRV-015 (Provention Bio/Sanofi), DONQ52 (Chugai Pharmaceuticals), KAN-101 (Kanyos Bio/Anokion), CALY-002 (Calypso Biotech/Novartis), and others are in development for celiac disease treatment.
The anticipated launch of these emerging celiac disease therapies is poised to significantly impact the market by offering new treatment options for a patient population that has been historically limited to a strict gluten-free diet. These innovative therapies, which aim to address the underlying immune response and improve symptom management, promise to enhance quality of life and increase treatment adherence among patients.
With better control over symptoms and the potential for disease modification, these therapies could also reduce the long-term complications associated with celiac disease, such as osteoporosis, infertility, and increased risk of certain cancers. As these therapies reach the market, they are likely to stimulate greater interest from both healthcare providers and patients, potentially expanding the market for celiac disease treatment options and leading to a shift in how the disease is managed.
Future of Celiac Disease Treatment Market Looks Promising
The future of the celiac disease treatment market is highly promising, driven by continuous advancements in medical research and an increasing understanding of the disease’s underlying mechanisms. Celiac disease, a chronic autoimmune disorder triggered by gluten, affects a significant portion of the global population, making it a priority for healthcare providers and researchers alike. As the prevalence of the condition rises, the demand for effective treatment options is expected to grow, moving beyond the traditional gluten-free diet. Innovations in enzyme therapies, immunotherapies, and drug development are creating new opportunities to manage and potentially cure the disease. For instance, treatments that focus on modulating the immune response to gluten, such as immunomodulators and peptide-based therapies, are showing encouraging results in clinical trials, potentially reducing the reliance on dietary restrictions and improving the quality of life for patients.
As a result, the celiac disease market is set for notable growth, fueled by rising awareness, better diagnostics, and increasing prevalence. In 2023, the United States held the largest share of the celiac disease market within the 7MM, accounting for approximately 70% of the total celiac disease treatment market. As diagnosis rates climb, demand for gluten-free products, targeted therapies, and advanced diagnostics is accelerating. Leading pharmaceutical companies, including Entero Therapeutics, Amgen, Takeda, and Sanofi, are actively developing innovative treatments to address unmet needs in this expanding celiac disease market.
In addition to pharmacological advances, diagnostic tools, and personalized treatment plans are also evolving, allowing for earlier and more accurate detection of celiac disease. With the development of companion diagnostics and tailored therapies, the market is poised for significant growth. Moreover, key players in the pharmaceutical and biotech industries are increasing their investment in research and development for celiac disease, further fueling innovation. The convergence of these technological and medical advancements, combined with growing patient awareness, is expected to result in a more robust and dynamic market. As treatment options become more effective and accessible, celiac disease patients can look forward to a future where managing the condition is less restrictive, more efficient, and potentially curative.

FAQs
The only approved treatment for celiac disease is a strict, lifelong gluten-free diet. This approach helps alleviate symptoms and promotes intestinal healing. However, many patients continue to experience symptoms due to accidental gluten exposure or incomplete adherence to the diet.
Some of the promising drugs in the celiac disease pipeline include Latiglutenase (First Wave BioPharma), TAK-101; TAK-227; Zamaglutenase (Takeda), PRV-015 (Provention Bio/Sanofi), DONQ52 (Chugai Pharmaceuticals), KAN-101 (Kanyos Bio/Anokion), CALY-002 (Calypso Biotech/Novartis), and others are in development for celiac disease treatment.
The celiac disease market is set for notable growth, fueled by rising awareness, better diagnostics, and increasing prevalence. In 2023, the United States held the largest share of the celiac disease market within the 7MM, accounting for approximately 70% of the total celiac disease treatment market.
Downloads
Article in PDF
Recent Articles
- Evaluating the Major Developments in the Smart Pills Market
- Celiac Disease: The Hidden Epidemic of Gluten Sensitivity
- Celiac Disease (CD) – Upcoming therapies to break the stagnancy
- Top 6 Celiac Disease Therapies Progressing in Mid-Stage Trials
- Which Pharma Companies are Working to Mitigate the Burden of Digestive Disorders?