Top 6 Celiac Disease Therapies Progressing in Mid-Stage Trials
Dec 06, 2024
Table of Contents
At present, there are no FDA-approved celiac disease therapies in the therapeutic segment. The only available treatment is a strict, lifelong gluten-free diet, which can be costly and challenging to follow. Many patients experience incomplete intestinal healing and persistent symptoms, often due to difficulties in maintaining full adherence to the diet. Promising celiac disease drug therapies are under development to ease the burden of managing celiac disease and enhance long-term health outcomes.
Read our latest article “Who Will Secure First-Mover Advantage in the Celiac Disease Treatment Space?” and get a complete overview
Downloads
Article in PDF
Recent Articles
- Which Pharma Companies are Working to Mitigate the Burden of Digestive Disorders?
- Celiac Disease: The Hidden Epidemic of Gluten Sensitivity
- Evaluating the Major Developments in the Smart Pills Market
- Celiac Disease Treatment: Who Will Secure First-Mover Advantage?
- Celiac Disease (CD) – Upcoming therapies to break the stagnancy
6 Celiac Disease Therapies in Mid-Stage Development
According to DelveInsight’s Celiac Disease Pipeline Report, over 25 companies are actively working in the celiac disease treatment field worldwide. Notable drugs in development include Latiglutenase (First Wave BioPharma), TAK-101, TAK-227, and Zamaglutenase (Takeda), as well as PRV-015 (Provention Bio/Sanofi), and KAN-101 (Kanyos Bio/Anokion), among others. Let’s take a closer look at these emerging celiac disease therapies in detail.
Sanofi’s PRV-015
PRV-015 (Ordesekimab, AMG 714) is a fully human IgG1κ monoclonal antibody being developed by Provention Bio for the treatment of Celiac Disease that does not respond to a gluten-free diet. Originally developed by Amgen for rheumatoid arthritis, the drug is currently undergoing a Phase II clinical trial (PROACTIVE) in adult patients with non-responsive celiac disease (NRCD) who are following a gluten-free diet. In March 2023, Sanofi acquired Provention Bio.
Takeda’s TAK-101; TAK-227; Zamaglutenase
Takeda is progressing with a portfolio of investigational therapies for potential celiac disease treatments. TAK-101 (CNP-101/TIMP-GLIA) is a first-in-class, immune-modulating biodegradable nanoparticle containing gliadin proteins, currently being developed by Takeda for celiac disease. The drug has received Fast Track designation from the US FDA for this condition. Another drug, Zamaglutenase (TAK-062), is in Phase II clinical trials to prevent gluten-specific T cell activation in patients with celiac disease following a gluten-free diet (GFD).
Additionally, TAK-227 (ZED1227), another celiac disease drug in Takeda’s pipeline, is in Phase IIb for celiac disease treatment. In October 2022, Takeda agreed with Zedira and Dr. Falk Pharma to further develop ZED1227/TAK-227. Zedira originally discovered the drug, and Dr. Falk Pharma obtained European rights in 2011, taking on responsibility for its preclinical and clinical development. Takeda expects to release Phase II results for TAK-227 by 2024.
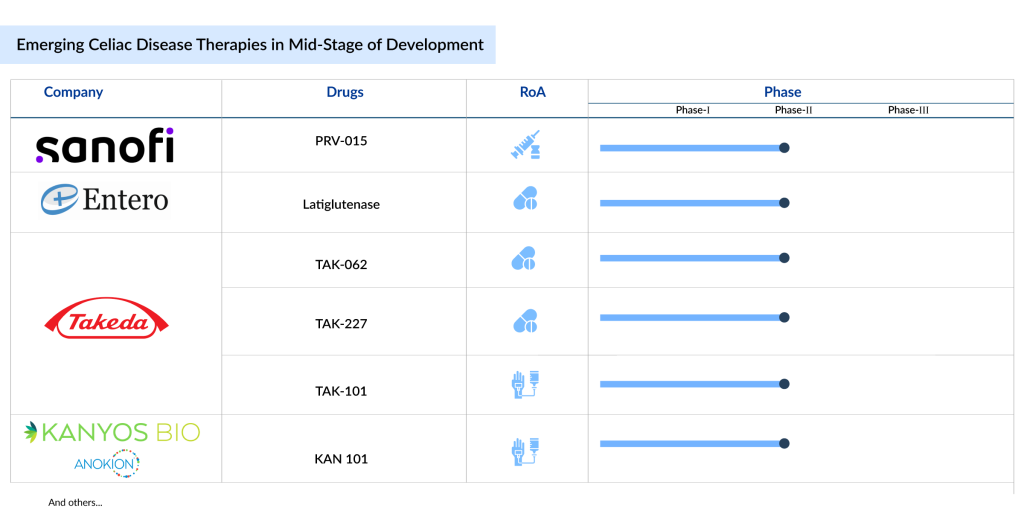
Entero Therapeutics’ Latiglutenase
Latiglutenase (IMGX003) is an oral treatment consisting of two gluten-specific recombinant proteases that break down gluten proteins into small, physiologically insignificant fragments. It is intended as an adjunct to a gluten-free diet (GFD). In Phase IIa and IIb clinical trials, latiglutenase demonstrated its ability to reduce gluten-induced intestinal mucosal damage and alleviate the severity and frequency of symptoms in patients with celiac disease.
The Phase III clinical development plan for latiglutenase has been reviewed by the GI Division of the US FDA, and the trials are expected to begin in early 2025. Additionally, a strategic US commercial licensing agreement with a global pharmaceutical company, along with concurrent institutional investment, is anticipated to be finalized in the second half of 2024.
Kanyos Bio/Anokion’s KAN 101
KAN-101 is an experimental therapy being tested as a potential treatment for celiac disease, a severe autoimmune disorder triggered by gluten consumption, for which no approved treatments currently exist. The therapy aims to promote tolerance to gliadin, a key component of gluten, by leveraging natural mechanisms in the liver. The FDA has granted KAN-101 Fast Track designation for the treatment of celiac disease.
In September 2023, Anokion announced clinical progress with KAN-101. The company has begun patient dosing in the global Phase II segment of the ACeD-it (Assessment of KAN-101 in Celiac Disease and Immune Tolerance) clinical trial, focusing on individuals with celiac disease. The ACeD-it study is composed of two phases: Phase Ib is an open-label, multiple ascending dose study to evaluate the safety and tolerability of higher doses of KAN-101 in celiac patients compared to the Phase I trial; Phase II is a double-blind, placebo-controlled trial aimed at assessing KAN-101’s impact on the gluten-induced IL-2 biomarker and celiac symptoms. KAN-101 is an innovative immune tolerance therapy using a gluten antigen delivered to the liver and immune system via the company’s proprietary liver-targeting technology.
The primary goal of the ACeD-it Phase II is to evaluate the effect on gluten-induced IL-2 biomarker responses. Key secondary objectives include measuring adverse events and pharmacokinetic parameters in patients treated with KAN-101, along with assessing symptom improvements in celiac disease following gluten exposure. The study will be conducted at sites in the US, Australia, and New Zealand. Anokion expects to report topline results in 2024.
Future Outlook of Celiac Disease Treatment
The future outlook for celiac disease treatment is promising, with ongoing research focusing on both therapeutic interventions and diagnostic advancements. Currently, the only treatment available is a strict gluten-free diet, but this does not fully address the disease’s underlying mechanisms. However, promising therapies are in development, including enzyme-based treatments that can help break down gluten in the digestive system, reducing the immune response. Oral immunotherapy is also being explored to desensitize the immune system to gluten exposure, potentially offering a long-term solution for patients who struggle with strict dietary management. Additionally, biologic drugs that target specific immune pathways responsible for celiac disease are being investigated, opening new avenues for treatment.
Another area of significant research involves enhancing diagnostic tools and expanding screening options for early detection of celiac disease. Improved biomarkers, blood tests, and genetic screening may lead to earlier intervention, preventing severe complications associated with untreated celiac disease, such as nutrient deficiencies, infertility, or autoimmune conditions. The development of a vaccine or therapy to allow for the safe consumption of gluten without triggering an immune response remains a long-term goal.
Additionally, several companies are evaluating their lead assets in various clinical trials. The introduction of these new therapies for celiac disease is set to have a major impact on the market by providing alternative treatment options for patients who have traditionally relied on a strict gluten-free diet. These groundbreaking therapies target the immune response and aim to improve symptom management, enhancing patients’ quality of life and encouraging better adherence to treatment.
With improved symptom control and the possibility of modifying the disease, these celiac disease therapies could help prevent long-term complications like osteoporosis, infertility, and an elevated risk of certain cancers. As these treatments become available, they are expected to spark increased interest from healthcare professionals and patients alike, potentially broadening the market for celiac disease treatments and changing the approach to managing the condition.
As science advances, these innovations, combined with personalized medicine, could significantly improve the quality of life for individuals with celiac disease and potentially offer a cure or more effective management options in the future.

Downloads
Article in PDF
Recent Articles
- Celiac Disease Treatment: Who Will Secure First-Mover Advantage?
- Celiac Disease: The Hidden Epidemic of Gluten Sensitivity
- Which Pharma Companies are Working to Mitigate the Burden of Digestive Disorders?
- Celiac Disease (CD) – Upcoming therapies to break the stagnancy
- Evaluating the Major Developments in the Smart Pills Market