AstraZeneca’s IMFINZI Archives Another Milestone — Becomes the First Immunotherapy for Limited-Stage Small-cell Lung Cancer
Dec 13, 2024
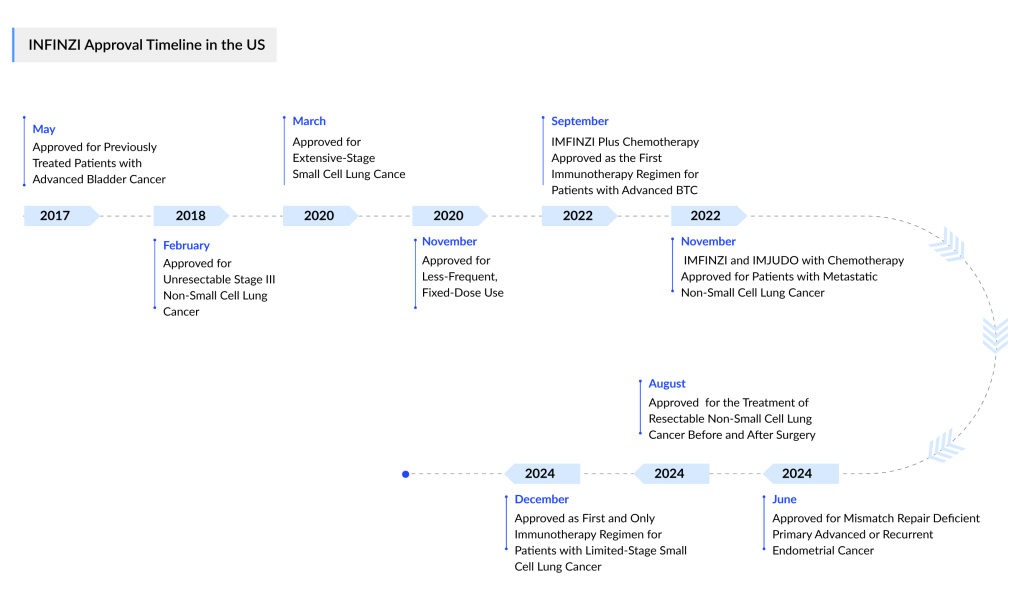
AstraZeneca has secured another approval for its cancer blockbuster IMFINZI (durvalumab), as the FDA has approved the PD-L1 inhibitor for patients with limited-stage small cell lung cancer (LS-SCLC) whose disease has not progressed following concurrent chemotherapy and radiation.
This approval makes IMFINZI the first immunotherapy available for LS-SCLC, a highly aggressive cancer subtype with a post-diagnosis survival rate of 15% to 30%. Lung cancer is the primary cause of cancer-related deaths in both men and women, responsible for roughly 20% of all cancer fatalities.
Limited-stage SCLC, referring to stages I-III, is typically confined to one lung or one side of the chest. LS-SCLC represents about 30% of all SCLC cases, and despite treatment with standard concurrent chemoradiotherapy (cCRT) aimed at cure, the prognosis remains poor. In 2023, there were 8.3K incident cases of LS-SCLC in the US, as per DelveInsight analysis.
Downloads
Article in PDF
Recent Articles
- Immuno-Oncology (I-O) Therapeutics: The Key to Future Cancer Treatment
- Rapid Global Expansion of Chinese PD-1/PD-L1 Key Players
- Merck’s Keytruda sales; Valeant on name change; Pfizer – BMS; Amgen puts Repatha outcomes for deal
- Genentech’s gantenerumab Fails in Phase III Trial; CHMP Recommends’ Dupixent; FDA Clears Imfinzi ...
- Pfizer & Lilly’s JAK Inhibitors Drug; FDA Approves Lilly’s Bebtelovimab; GSKR...
The FDA approved the treatment following Priority Review and Breakthrough Therapy Designation. This decision was supported by findings from the ADRIATIC Phase III trial, which were highlighted during the Plenary Session of the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting and later published in the New England Journal of Medicine.
In the trial, IMFINZI demonstrated a 27% reduction in the risk of death compared to the placebo (overall survival [OS] hazard ratio [HR]: 0.73; 95% confidence interval [CI]: 0.57-0.93; P=0.0104). The estimated median OS was 55.9 months for IMFINZI, compared to 33.4 months for placebo, with 57% of IMFINZI-treated patients alive at three years versus 48% for placebo.
Additionally, IMFINZI reduced the risk of disease progression or death by 24% (progression-free survival [PFS] HR: 0.76; 95% CI: 0.61-0.95; P=0.0161) compared to placebo. Median PFS was 16.6 months for IMFINZI versus 9.2 months for placebo. At two years, 46% of patients receiving IMFINZI showed no disease progression compared to 34% for placebo.
The safety profile of IMFINZI was generally manageable and aligned with its known characteristics, with no new safety concerns identified. IMFINZI has also been approved for this indication in Switzerland based on the ADRIATIC trial results, and regulatory reviews are underway in the EU, Japan, and other countries.
IMFINZI is a human monoclonal antibody that targets the PD-L1 protein, blocking its interaction with PD-1 and CD80, thereby counteracting the tumor’s ability to evade the immune system and enabling immune responses to be activated.
In addition to its approval for LS-SCLC, IMFINZI is the only immunotherapy authorized as the global standard of care for curative treatment in unresectable, Stage III NSCLC patients whose disease has not progressed after chemoradiation therapy (CRT). IMFINZI is also approved as a perioperative treatment alongside neoadjuvant chemotherapy for resectable NSCLC, in combination with chemotherapy (etoposide and either carboplatin or cisplatin) for extensive-stage SCLC, and with a short course of tremelimumab-actl and chemotherapy for metastatic NSCLC.
IMFINZI is further approved in combination with chemotherapy (gemcitabine and cisplatin) for locally advanced or metastatic biliary tract cancer and with tremelimumab-actl for unresectable hepatocellular carcinoma (HCC). In Japan and the EU, IMFINZI is approved as a monotherapy for unresectable HCC.
In the US, IMFINZI is authorized in combination with chemotherapy (carboplatin and paclitaxel) followed by IMFINZI monotherapy for primary advanced or recurrent endometrial cancer with mismatch repair deficiency (dMMR). In the EU, IMFINZI combined with chemotherapy, followed by olaparib and IMFINZI, is approved for advanced or recurrent endometrial cancer with proficient mismatch repair (pMMR), and IMFINZI with chemotherapy, followed by IMFINZI alone, is approved for dMMR cases. Japan has approved IMFINZI with chemotherapy, followed by IMFINZI monotherapy, as first-line treatment for primary advanced or recurrent endometrial cancer, and a combination of IMFINZI, chemotherapy, and olaparib for pMMR patients.
IMFINZI is also under global regulatory review as a perioperative treatment in combination with neoadjuvant chemotherapy, based on the NIAGARA Phase III trial, which showed a significant and clinically meaningful improvement in event-free survival and overall survival compared to neoadjuvant chemotherapy alone.
Since its approval in May 2017, over 374,000 patients have been treated with IMFINZI. As part of an extensive development program, IMFINZI is being investigated both as a monotherapy and in combination with other anti-cancer treatments for patients with SCLC, NSCLC, breast cancer, bladder cancer, various gastrointestinal and gynecologic cancers, and other solid tumors.
IMFINZI is a cornerstone of AstraZeneca’s oncology portfolio. Out of AstraZeneca’s $37.6 billion revenue in the first three quarters of this year, over $16 billion came from oncology products. While NSCLC leader TAGRISSO contributed $4.9 billion in sales through Q3, IMFINZI brought in $3.5 billion.
With this approval, IMFINZI will become a cornerstone in the treatment of LS-SCLC, and its success could shift clinical practice guidelines, leading to wider adoption of immunotherapy combinations. This may, in turn, increase the market share for immunotherapies in both ES-SCLC and LS-SCLC, potentially changing the standard of care for these patients.
In response, other pharmaceutical companies are intensifying their research and development efforts to introduce competitive therapies. For instance, trials are underway to evaluate the efficacy of combining immunotherapies with traditional treatments like chemotherapy and radiation, aiming to enhance patient outcomes.
Currently, Amgen is evaluating its lead product, Tarlatamab, for LS-SCLC. Once approved, it may be a potential contender to IMFINZI in the LS-SCLC therapeutic segment. IMDELLTRA is a bispecific T cell engager (BiTE) molecule that targets both delta-like ligand 3 (DLL3) and CD3. It is being studied as a potential treatment for small-cell lung cancer. In May 2024, Amgen announced that the FDA had approved IMDELLTRA for use in adult patients with extensive-stage small cell lung cancer whose disease has progressed after platinum-based chemotherapy.
Additionally, studies are exploring the use of immune checkpoint inhibitors, such as nivolumab and ipilimumab, in combination with chemoradiation therapy for LS-SCLC patients. These initiatives reflect a broader industry commitment to improving treatment efficacy and patient survival in LS-SCLC.
Furthermore, there may be increased collaboration and partnerships within the industry to enhance the efficacy and safety profiles of emerging therapies. The approval of IMFINZI not only provides a new treatment avenue for LS-SCLC patients but also sets a precedent for future therapeutic innovations in this challenging area of oncology.

Downloads
Article in PDF
Recent Articles
- X4 Pharmaceuticals’ XOLREMDI FDA Approval; ONO to Acquire Deciphera Pharmaceuticals; Johnson &...
- FDA Grants Priority Review to BMS’ Luspatercept; Teva and MedinCell’s Risperidone FDA Approval; B...
- Potential of EGFR Inhibitors: A Promising Avenue in Cancer Treatment
- Astellas & AviadoBio’s Exclusive Deal for AVB-101; GSK’s Depemokimab Shows Positive Res...
- Nkarta’s Anti-CD19 Allogeneic CAR-NK Cell Therapy, NKX019; Eisai Presents Results of lecanemab fo...