Merus’ BIZENGRI: The New Face of NRG1+ Cancer Treatment After FDA Approval
Dec 16, 2024
Merus had a standout start this month. Shortly after landing a long-anticipated commercialization partner for its zenocutuzumab, the drug has achieved FDA approval, becoming the first therapy to target cancers with a neuregulin 1 (NRG1) gene fusion.
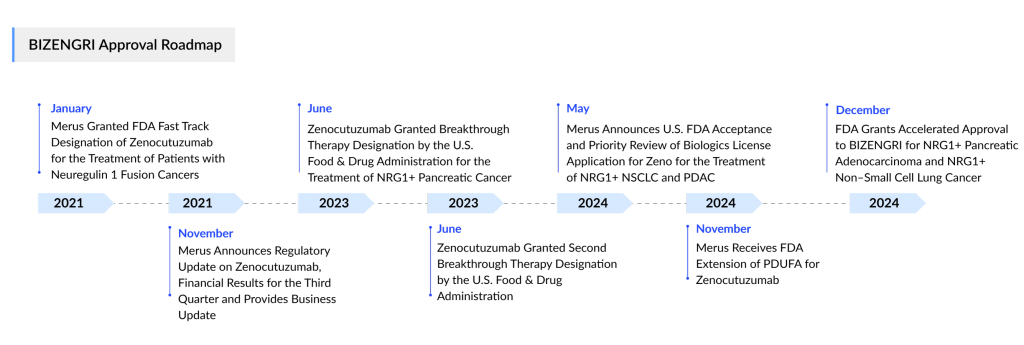
The FDA granted accelerated approval to the drug, now marketed as BIZENGRI, for treating NRG1 fusion-positive non-small cell lung cancer (NSCLC) and NRG1 fusion-positive pancreatic adenocarcinoma in patients whose disease has progressed after previous systemic therapy.
NRG fusions have been identified in over ten types of solid tumors, indicating they represent a potential target for a wide, tumor-agnostic therapeutic strategy. NRG1 rare fusions are found in various solid tumors at a frequency of 0.1%-0.5%, with a higher prevalence in cancers such as NSCLC and pancreatic cancer.
Downloads
Article in PDF
Recent Articles
- Astellas & AviadoBio’s Exclusive Deal for AVB-101; GSK’s Depemokimab Shows Positive Res...
- CDK 4/6 Inhibitors- The Changing Paradigm for Breast Cancer Treatment
- ImPact Biotech’s IND Application for Padeliporfin VTP; Orphan Drug Designation to Ocelot Bio’s OC...
- 6 Upcoming Bispecific Antibodies Beyond Oncology
- Unleashing the Potential: CD38 Directed Therapies Revolutionize Multiple Myeloma Treatment
DelveInsight’s epidemiology analysis estimates the patient burden of NRG1 fusions across several cancers, including cholangiocarcinoma, pancreatic cancer, renal cell carcinoma, ovarian carcinoma, NSCLC, breast cancer, bladder cancer, and colorectal cancer. In 2023, there were over 3,000 incident cases of NRG1 fusion in the US, and this number is projected to increase by 2034.
The FDA had previously postponed its decision on the therapy to assess additional information related to chemistry, manufacturing, and controls. Initially, the agency was expected to announce its approval decision by early November.
In the meantime, Merus had been searching for a commercialization partner since at least 2023, describing such an agreement as a critical step toward making the bispecific therapy available to patients with NRG1-positive cancer.
On 2 December 2024, Merus announced a partnership with Partner Therapeutics, granting the Massachusetts-based company an exclusive U.S. commercialization license. In return, Merus will receive an undisclosed upfront payment, potential milestone payments, and royalties ranging from high single digits to low double digits based on annual net sales.
“The FDA’s approval of BIZENGRI represents a significant advancement for patients with advanced, unresectable, or metastatic pancreatic adenocarcinoma or NSCLC that carries the NRG1 gene fusion. I have personally witnessed how BIZENGRI® treatment can provide meaningful clinical outcomes for patients,” stated Alison Schram, MD, an attending medical oncologist in the Early Drug Development Service at Memorial Sloan Kettering Cancer Center and principal investigator for the ongoing eNRGy trial. “I am deeply thankful to the patients and families who were part of the trial.”
The approval of BIZENGRI is supported by results from the eNRGy trial, a multicenter, open-label clinical study involving patients with advanced, unresectable, or metastatic NRG1+ pancreatic adenocarcinoma or NRG1+ NSCLC who experienced disease progression after prior systemic treatment. Among patients with NRG1+ pancreatic adenocarcinoma (n=30), BIZENGRI achieved an objective response rate (ORR) of 40% (95% CI, 23%-59%), with a duration of response (DOR) ranging from 3.7 to 16.6 months.
In the same trial, patients with NRG1+ NSCLC (n=64) demonstrated an ORR of 33% (95% CI, 22%-46%) and a median DOR of 7.4 months (95% CI, 4.0-16.6). Responses were evaluated using RECIST v1.1 criteria and assessed by blinded independent central review (BICR).
In the pooled safety analysis (N=175), the most frequent adverse reactions (≥10%) included diarrhea, musculoskeletal pain, fatigue, nausea, infusion-related reactions, dyspnea, rash, constipation, vomiting, abdominal pain, and edema.
Common Grade 3 or 4 laboratory abnormalities (≥2%) included increased gamma-glutamyltransferase, decreased hemoglobin, decreased sodium, decreased platelets, increased aspartate aminotransferase, increased alanine aminotransferase, increased alkaline phosphatase, decreased magnesium, decreased phosphate, prolonged activated partial thromboplastin time, and increased bilirubin.
“BIZENGRI is the first medicine from Merus to receive approval, leveraging our groundbreaking and proprietary Biclonics technology platform. It holds significant potential for patients with NRG1+ pancreatic adenocarcinoma and NRG1+ NSCLC,” stated Shannon Campbell, Chief Commercial Officer of Merus. “This milestone highlights the strength of our technology and our ability to deliver, as we advance the development of our multispecific platforms and pipeline, including our leading asset, petosemtamab.”
BIZENGRI is a bispecific antibody designed to target the extracellular domains of HER2 and HER3 on cell surfaces, including tumor cells. It inhibits HER2:HER3 dimerization and blocks NRG1 from binding to HER3. By doing so, BIZENGRI reduces cell proliferation and disrupts signaling via the phosphoinositide 3-kinase-AKT-mTOR pathway. Additionally, it induces antibody-dependent cellular cytotoxicity. In preclinical studies, BIZENGRI demonstrated antitumor activity in mouse models of NRG1-positive lung and pancreatic cancers.
BIZENGRI carries a boxed warning highlighting the risk of embryo-fetal toxicity. Merus expects the drug to be available for intravenous administration in the coming weeks. However, as it received accelerated approval, its efficacy will need to be validated through confirmatory trials.
The company aims to assist eligible patients in accessing BIZENGRI by offering resources and support tailored to their individual needs and circumstances. PTx Assist is available to guide patients through the treatment process, provide educational materials, help with insurance coverage understanding, and identify possible financial assistance options.
The BIZENGRI approval has heated the competition in the NRG fusion space. As a result, the NRG fusion market is anticipated to grow at a CAGR of 15% due to the first launch of Merus’ BIZENGRI and increased adoption of NRG1 fusion biomarker testing during the study period (2020–2034).
Additionally, companies such as Hummingbird Bioscience (HMBD-001), Salubris Biotherapeutics (Seribantumab), and others are developing novel NRG fusion therapies. However, in 2023, Elevation Oncology halted the development of Seribantumab for NRG1 fusions (CRESTONE trial) and indicated that they would resume its development only in partnership with a collaborator, making the future of the trial uncertain at this time.
On the other hand, even though in the early stage of development, Hummingbird Bioscience is also focussing on pancreatic cancer and NSCLC patients harboring NRG1 fusions along with solid tumors harboring NRG1 fusion and HER3 extracellular mutation in a Phase IB trial.
The anticipated launch of these emerging therapies are further poised to transform the NRG fusion market landscape in the coming years. It will be interesting to see how BIZENGRI will maintain its monopoly in the NRG1 fusion therapeutic segment until it gets competition from other emerging therapies.

Downloads
Article in PDF
Recent Articles
- Unveiling the Potential of TROP-2 Inhibitors: A New Frontier in Cancer Treatment
- Non-Small Cell Lung Cancer Market: Treatments and Market Forecast
- Lilly’s Morphic Acquisition; IDEAYA’s IDE397 Positive Phase II Trial Result; XPOVIO (seline...
- Evaluating Key Advancements and Emerging Therapies in EGFR-Non Small Cell Lung Cancer Treatment M...
- Copiktra receives approval; Lilly wins approval; Eisai’s Fycompa; Astellas’ Roxadustat; Janssen’s...