Thyroid Eye Disease: The Hidden Impact of Thyroid Dysfunction on Eye Health
Jan 20, 2025
Table of Contents
Thyroid eye disease is a rare but serious autoimmune condition that affects the eyes, often linked to hyperthyroidism or Graves’ disease. Around 25-50% of people with Graves’ disease develop TED, with women being more commonly affected than men.
There were 1.4 million diagnosed prevalent cases of TED in 2023, with the number projected to rise by 2034, as per DelveInsight’s Thyroid Eye Disease Epidemiology Forecast Report. In the 7MM, the US had the highest number of diagnosed prevalent cases of TED
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Ipsen to Acquire Albireo; Chiesi Farmaceutici to Buy Amryt Pharma; Takeda Presents Phase III Resu...
- Graves’ Ophthalmopathy Treatment Market: A Billion-Dollar Opportunity For Pharma Companies
- Graves’ Disease Treatment Revolution: What’s Next in Line?
- Thyroid awareness month
- Tepezza receives approval; a new way to treat Alzheimer’s
In 2023, the US reported the maximum number of mild cases, followed by moderate to severe cases, and sight-threatening cases of TED. These numbers are expected to rise during the forecast period from 2024 to 2034. The higher prevalence of early-stage cases across all forms of TED can be attributed to improved awareness, earlier detection, and better diagnostic tools. Additionally, efforts to reduce the stigma surrounding the condition have led to increased reporting of cases at all levels of severity.
TED can significantly impact a person’s quality of life, causing symptoms like dryness, redness, pain, and even vision problems. Early diagnosis and treatment are crucial to managing the disease and preventing long-term damage, emphasizing the need for greater awareness and research in this area.
Read our blog on Graves’ Disease Treatment to understand the difference between thyroid eye disease and Graves’ disease
TEPEZZA — Only Approved Drug for Thyroid Eye Disease Treatment
Teprotumumab is a fully human IgG1 monoclonal antibody that inhibits the insulin-like Growth Factor-1 receptor (IGF-1R). It is produced in Chinese hamster ovary (CHO-DG44) cells and has a molecular weight of approximately 148 kDa.
In January 2020, the US FDA granted accelerated Priority Review approval for TEPEZZA as a treatment for thyroid eye disease. More recently, in September 2024, Japan’s Ministry of Health, Labour, and Welfare (MHLW) approved the drug for treating active Graves’ orbitopathy. Furthermore, in April 2024, Amgen announced its intent to submit a Marketing Authorization Application (MAA) for teprotumumab to the European Medicines Agency (EMA) in the near future.
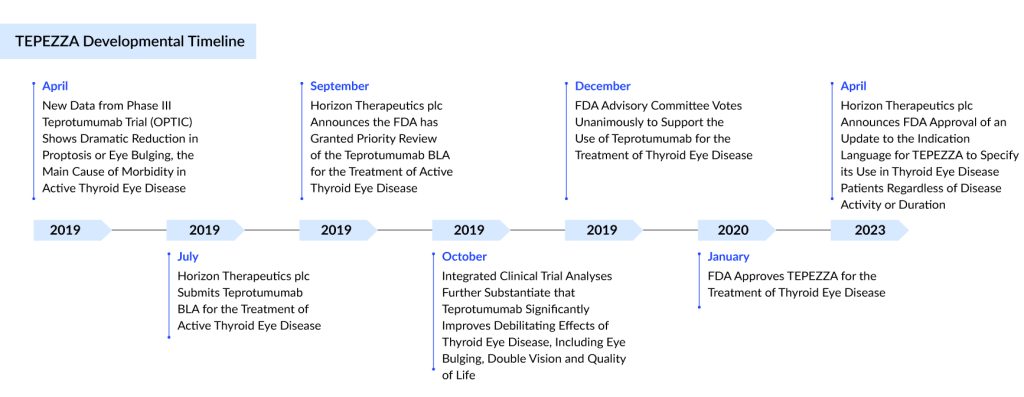
Currently, TEPEZZA is being evaluated in Phase III clinical trials for moderate-to-severe active Graves’ orbitopathy and chronic TED with a low Clinical Activity Score (CAS). Amgen is also investigating the feasibility of subcutaneous administration of the drug.
However, with only one drug currently approved in the thyroid eye disease arena, there is a significant opportunity for companies to enter the thyroid eye disease treatment market.
Understand more about the evolving landscape of Graves’ Ophthalmopathy by reading our article on Graves’ Ophthalmopathy Treatment
Promising Thyroid Eye Disease Drugs in Line for Approval
The thyroid eye disease treatment pipeline is robust. Various potential therapies that are projected to enter the market during the forecast period include Viridian Therapeutics’ VRDN-001(veligrotug), Argenx’ Efgartigimod PH20 SC, Hoffmann-La Roche’ ENSPRYNG (satralizumab, RG6168), Sling Therapeutics’ Linsitinib, Tourmaline Bio’ Pacibekitug (TOUR006), Lassen Therapeutics’ LASN01, ACELYRIN’ Lonigutamab, and Immunovant, Samsung Biologics, HanAll Biopharma and Roivant Sciences’ IMVT-1401 (batoclimab, RVT-1401).
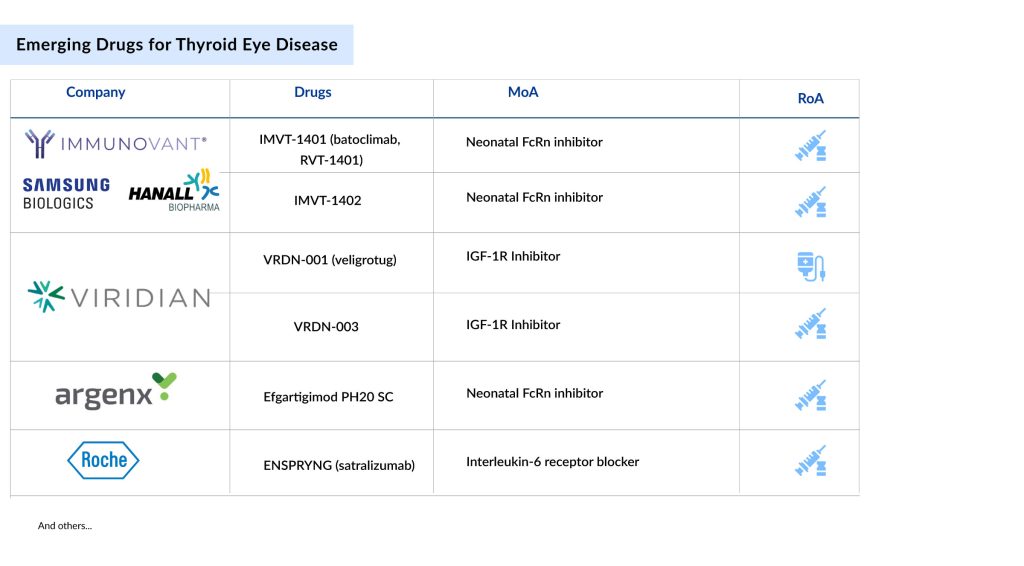
The anticipated launch of these therapies from different pharmaceutical companies are set to compete with Amgen’s market-leader TEPEZZA in the treatment of thyroid eye disease. These innovative treatments utilize advanced approaches, including targeted biologics and novel small molecules, which could provide benefits in terms of effectiveness, safety, and patient convenience. For example, certain emerging therapies for thyroid eye disease target specific inflammatory pathways or employ cutting-edge delivery systems to enhance therapeutic results.
Get detailed insights into the late-stage TED drugs at “Late-Stage Thyroid Eye Disease Treatments: 4 Prominent Therapies to Consider”
Does Sling’s Oral Linsitinib a Potential Contender to Amgen’s Intravenous TEPEZZA?
Recently in January 2025, Sling Therapeutics’ oral alternative to Amgen’s intravenous TEPEZZA has shown positive results in a phase IIb/III clinical trial for thyroid eye disease, positioning the company to conduct a confirmatory study of its ex-Astellas asset. However, a comparison across trials indicates that Amgen’s widely successful drug may have an edge in terms of efficacy.
Astellas previously investigated the small-molecule IGF-1R inhibitor linsitinib as a cancer treatment. With TEPEZZA, an anti-IGF-1R antibody, demonstrating positive effects in TED, Sling saw an opportunity to explore linsitinib for the condition. The company began its efforts in 2022, securing $35 million to run a phase IIb trial in TED, a complication of Graves’ disease that leads to eye bulging.
About 18 months later, Sling has gathered evidence supporting linsitinib’s efficacy in TED. In the trial, 90 patients were randomized to receive either one of two doses of linsitinib or a placebo. After 24 weeks, 52% of those on the higher dose showed a significant reduction in eye bulging, meeting the response criteria.
The high dose response rate was notably higher than the placebo group, allowing it to meet the primary endpoint. Sling did not provide information on the performance of the lower dose and is now preparing to initiate a confirmatory phase III trial later this year.
Safety and tolerability could be another key factor. The upcoming phase III trial will provide further insights into the molecule’s safety and efficacy, potentially positioning Sling to compete with Amgen in the TED market.
Recent Developments in the Graves’ Disease Treatment Space
- In January 2025, Sling Therapeutics announced promising Phase IIb/III results for linsitinib in treating thyroid eye disease, potentially paving the way for the first oral option to Amgen’s intravenous TEPEZZA in a market that analysts believe is still largely untapped.
- In August 2024, ACELYRIN decided to pivot its focus from izokibep, despite the drug’s continued strong performance in clinical trials. The company laid off around 40 employees—approximately 33% of its 135-person workforce—to prioritize the development of lonigutamab, a potential competitor to Amgen’s blockbuster drug, TEPEZZA.
- According to Immunovant’s Q3 2024 annual report released in November, the company expects data from batoclimab trials in TED to be available in the second half of 2025. Additionally, a June 2024 SEC filing revealed that, following a recent Type B meeting with the US FDA, the company plans to launch four to five potentially registrational programs for IMVT-1402 across various therapeutic areas, including endocrinology and neurology, by March 2025.
- Viridian Therapeutics aims to submit a Biologics License Application (BLA) for veligrotug in TED treatment by the second half of 2025. The company also expects topline data from the REVEAL-1 and REVEAL-2 trials of VRDN-003, targeting active and chronic TED, in the first half of 2026.
- In August 2023, Tourmaline Bio announced that the US FDA had approved its IND application for TOUR006.
Thyroid Eye Disease Treatment: The Road Ahead
In the coming years, the US thyroid eye disease market is set for significant change and growth, largely driven by the dominance of the already approved product, TEPEZZA. Additionally, we expect the launch of a second product, batoclimab, in the US within the next 2–3 years. Since only about 20% of thyroid eye disease cases are acute and treated, we anticipate market expansion, particularly with the introduction of safer and more effective therapies.
Currently, the market size of TED in the 7MM was USD 2.3 billion in 2023, which is further expected to increase by 2034, as per DelveInsight’s Thyroid Eye Disease Market Forecast Report. This growth is mainly driven by increasing awareness about TED may increase market size in the coming years, assisted by an increase in the prevalence of TED.
Additionally, the arrival of new treatments, along with improved early patient screening, the integration of medications in secondary care settings, ongoing research on best implementation practices, and increased awareness, will contribute to the development of more effective treatment options. However, challenges remain in the timely diagnosis and treatment of patients, especially as the condition is classified as a rare disease. This rarity leads companies to set premium prices, which can be a barrier to the adoption of new therapies.

Downloads
Article in PDF
Recent Articles
- Thyroid eye disease market: New therapies enter the TED market
- Ipsen to Acquire Albireo; Chiesi Farmaceutici to Buy Amryt Pharma; Takeda Presents Phase III Resu...
- Late-Stage Thyroid Eye Disease Treatments: 4 Prominent Therapies to Consider
- Graves’ Ophthalmopathy Treatment Market: A Billion-Dollar Opportunity For Pharma Companies
- Graves’ Disease Treatment Revolution: What’s Next in Line?