Graves’ Disease Drug Pipeline: 6 Late-Stage Therapies to Watch
Jan 24, 2025
The pipeline for Graves’ disease drug treatments is rapidly expanding, with prominent companies such as Immunovant, Viridian Therapeutics, Argenx, Tourmaline Bio, Hoffmann-La Roche, and Sling Therapeutics spearheading the development of targeted therapies for thyroid disorders. Promising candidates in the late stages of Graves’ disease treatment include veligrotug (VRDN-001), VRDN-003, batoclimab, Efgartigimod PH20 SC, ENSPRYNG (satralizumab, RG6168), and IMVT-1402.
These emerging therapies, which target various mechanisms and molecular pathways, offer innovative alternatives to current treatments. By providing novel approaches to managing autoimmune conditions such as thyroid eye disease, they highlight an increasing emphasis on more precise and targeted solutions for these complex disorders.
For more insights into Graves’ disease therapeutic segment, read our blog “Graves’ Disease Treatment Revolution: What’s Next in Line?”
Downloads
Article in PDF
Recent Articles
- Graves’ Disease Treatment Revolution: What’s Next in Line?
- Late-Stage Thyroid Eye Disease Treatments: 4 Prominent Therapies to Consider
- Ipsen to Acquire Albireo; Chiesi Farmaceutici to Buy Amryt Pharma; Takeda Presents Phase III Resu...
- FDA Approves Xolair for Food Allergies; FDA Accelerated Approval for Iovance’s AMTAGVI; Astellas ...
- Thyroid awareness month
Now, let’s look at the 6 promising late-stage therapies for Graves’ disease that will be launched in the coming years.
Immunovant, Samsung Biologics, HanAll Biopharma, and Roivant Sciences’ IMVT-1401 and IMVT-1402
Immunovant’s pipeline features two advanced anti-FcRn monoclonal antibodies, IMVT-1402 and batoclimab (formerly IMVT-1401), both developed for subcutaneous administration with flexible dosing tailored to disease severity. These therapies target the neonatal Fc receptor to lower pathogenic IgG antibodies, addressing conditions associated with elevated IgG levels.
Immunovant’s lead investigational product, batoclimab (IMVT-1401), is a novel, fully human monoclonal antibody designed to target the neonatal Fc receptor (FcRn). Research from nonclinical studies and clinical trials has shown that batoclimab can lower IgG antibody levels, which are often elevated in various autoimmune diseases. By reducing pathogenic IgG antibodies, this candidate therapy has the potential to treat a range of IgG-mediated autoimmune conditions as a self-administered subcutaneous (SC) injection.
IMVT-1402 is specifically designed to avoid the albumin reductions and LDL cholesterol increases observed with batoclimab while maintaining comparable IgG-lowering efficacy. Drawing from insights gained in ongoing batoclimab studies, Immunovant is fast-tracking the development of IMVT-1402 in critical areas such as rheumatology, endocrinology, and neurology, targeting diseases like Graves’ disease, myasthenia gravis, CIDP, and rheumatoid arthritis.
Phase II data for batoclimab in Graves’ disease demonstrate the potential of FcRn inhibition to counter disease pathology driven by thyroid-stimulating hormone receptor autoantibodies, with greater IgG reductions linked to improved clinical outcomes.
Currently, batoclimab is being developed as a low-volume SC injection to address autoimmune disorders such as myasthenia gravis, thyroid eye disease, CIDP, and Graves’ disease.
In September 2024, Immunovant secured Investigational New Drug (IND) clearance from the US FDA for IMVT-1402 to treat Graves’ disease patients who remain hyperthyroid despite using antithyroid drugs. The company also plans to launch clinical trials of IMVT-1402 across ten indications by March 2026.
Viridian Therapeutics’ VRDN-001 and VRDN-003
Viridian Therapeutics distinguishes itself as the only company developing therapies for both moderate-to-severe active TED and chronic TED. Since chronic TED significantly contributes to the overall disease burden, addressing this segment could enable the company to secure a considerable market share.
Viridian is advancing a robust IGF-1R antibody portfolio for TED, with its lead candidate, veligrotug (formerly VRDN-001), an IV-administered monoclonal antibody. VRDN-001 is a unique monoclonal antibody targeting the Insulin-like Growth Factor-1 Receptor (IGF-1R), a clinically and commercially established target for treating Graves’ ophthalmopathy. Preclinical studies demonstrated that VRDN-001 acts as a full IGF-1R antagonist, achieving complete receptor blockade, surpassing other anti-IGF-1R antibodies, including the currently approved therapy for Graves’ ophthalmopathy.
Early data from Phase II trial cohorts confirmed the clinical proof-of-concept for VRDN-001 in patients with active Graves’ ophthalmopathy. Preliminary results showed that VRDN-001 treatment significantly reduced proptosis, improved CAS scores, and resolved diplopia. The therapy was generally safe and well-tolerated throughout the trial.
In September 2024, veligrotug demonstrated strong results in the Phase III THRIVE trial, achieving primary and secondary endpoints with high statistical significance. The treatment showed rapid efficacy, with 53% of patients experiencing a proptosis response after just one infusion, and it was well tolerated, with no treatment-related serious adverse events reported. Topline data from the THRIVE-2 trial (focused on chronic TED) is anticipated by the end of 2024, and a BLA submission is planned for the second half of 2025.
VRDN-003 is also an IGF-1R antibody that shares the same binding domain as veligrotug. It is believed to be the only anti-IGF-1R therapy in development with an extended half-life. Viridian considers the topline results from the THRIVE study to strongly support a potential best-in-class profile for VRDN-003. This therapy offers clinical efficacy and safety comparable to veligrotug in a low-volume, infrequent, self-administered subcutaneous injection suitable for at-home use.
The pivotal REVEAL trials (REVEAL-1 for active TED and REVEAL-2 for chronic TED) are currently in progress. Topline results are anticipated in the first half of 2026, followed by the planned BLA submission by year-end. With Veligrotug and VRDN-003, the company is poised to establish itself as a leader in the TED market, offering both intravenous and subcutaneous delivery options.
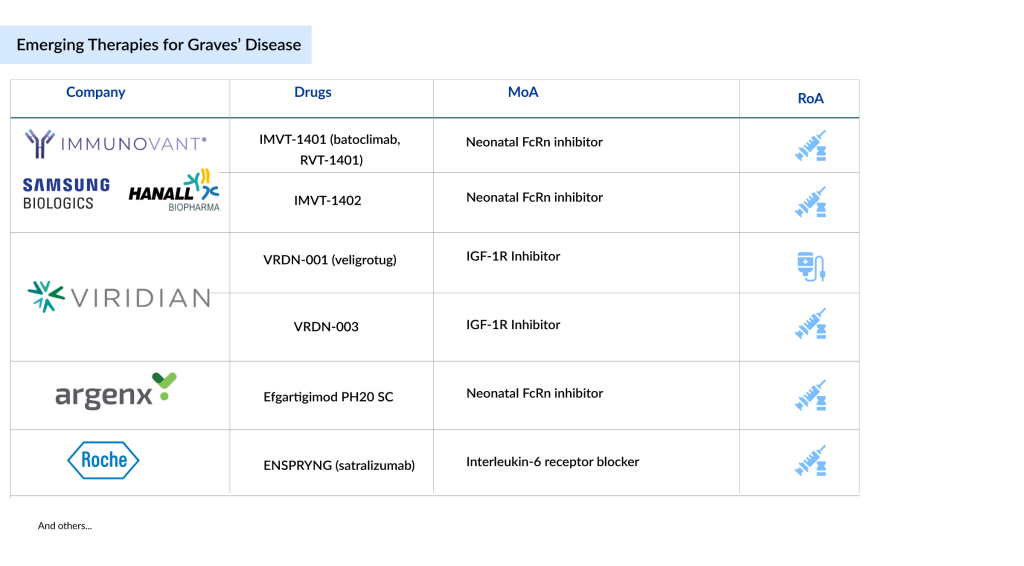
Argenx’s Efgartigimod PH20 SC
Efgartigimod PH20 SC, developed by Argenx, is an experimental treatment for thyroid eye disease. This fully human antibody fragment targets the neonatal Fc receptor (FcRn) to inhibit its function. By blocking FcRn, the drug lowers the levels of harmful immunoglobulin G (IgG) autoantibodies, which are thought to drive autoimmune conditions.
Currently in Phase III proof-of-concept trials, the subcutaneous formulation (PH20 SC) is designed to enhance drug dispersion and absorption, potentially boosting its effectiveness and ease of use for patients. With its novel mechanism of action and user-friendly administration, Efgartigimod PH20 SC offers promising benefits over existing treatments. Argenx is advancing this thyroid eye disease drug toward regulatory approval to provide a new, effective solution for managing TED.
Hoffmann-La Roche’s ENSPRYNG
ENSPRYNG (satralizumab, RG6168), developed by Roche, is a humanized monoclonal antibody designed to target the interleukin-6 (IL-6) receptor. IL-6 is a signaling protein produced by immune cells that plays a role in autoimmune disorders, including TED. Currently, in Phase III trials for TED, Roche is working to progress ENSPRYNG toward regulatory approval, aiming to offer a new treatment option for this chronic condition.
The anticipated launch of emerging therapies for Graves’ disease is poised to significantly reshape its treatment landscape, offering new hope to patients and addressing unmet clinical needs. Traditional therapies, including antithyroid medications, radioactive iodine, and thyroidectomy, often come with limitations such as incomplete remission, recurrence, or long-term side effects.
These novel Graves’ disease drugs could reduce reliance on invasive procedures and improve the quality of life for patients by minimizing disease relapse and adverse effects. Furthermore, their introduction is likely to spur competition and innovation within the market, encouraging further research into personalized approaches and combination regimens, ultimately elevating the standard of care for Graves’ disease.

Downloads
Article in PDF
Recent Articles
- Late-Stage Thyroid Eye Disease Treatments: 4 Prominent Therapies to Consider
- Thyroid Eye Disease: The Hidden Impact of Thyroid Dysfunction on Eye Health
- Graves’ Disease Treatment Revolution: What’s Next in Line?
- Thyroid awareness month
- Ipsen to Acquire Albireo; Chiesi Farmaceutici to Buy Amryt Pharma; Takeda Presents Phase III Resu...