The Future of Multiple System Atrophy Treatment: Pioneering Approaches Beyond Levodopa Reliance
Jan 27, 2025
Table of Contents
Multiple system atrophy (MSA) is a rare and progressive neurodegenerative disorder that affects the autonomic nervous system, motor function, and balance. It manifests through a combination of parkinsonism, autonomic dysfunction, and cerebellar ataxia, making it distinct from Parkinson’s disease despite overlapping symptoms. Unlike Parkinson’s, MSA progresses rapidly, and available treatments focus mainly on symptom relief rather than addressing the root causes of the disease, leaving significant unmet medical needs.
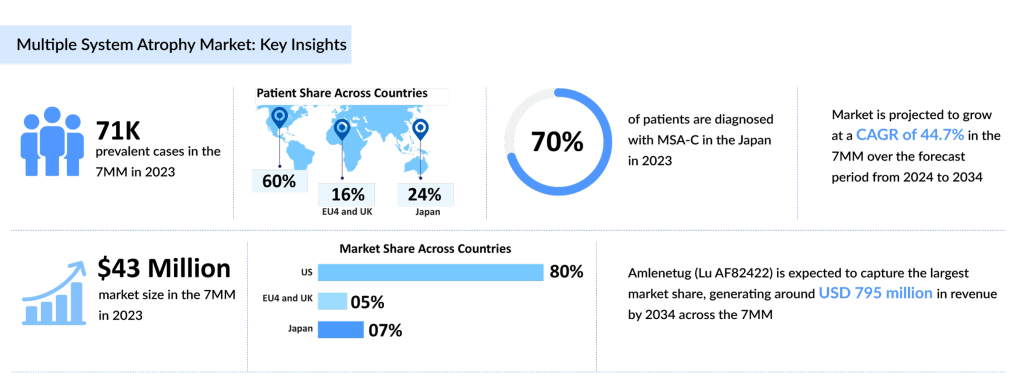
As per DelveInsight’s analysis, approximately 70,000 people across the seven major markets (the United States, Germany, France, Italy, Spain, the United Kingdom, and Japan) are diagnosed with MSA in 2023, with the US accounting for approximately 60% of the cases, highlighting the need for accelerated research and therapeutic innovation.
MSA presents in two primary subtypes—MSA-P (parkinsonian) and MSA-C (cerebellar)—each shaping the patient’s clinical course in distinct ways. MSA-P, marked by rigidity and tremors resembling Parkinson’s disease, can complicate diagnosis, while MSA-C predominantly affects balance and coordination, manifesting as cerebellar ataxia.
Additionally, it is noteworthy that in the US and European countries, including Germany, France, Italy, Spain, and the United Kingdom, an average of 70% of patients are diagnosed with MSA-P, while 30% have MSA-C. In contrast, Japan shows a different distribution, with 30% of cases being MSA-P and 70% MSA-C, possibly due to regional differences in disease expression and genetic factors.
Furthermore, MSA progresses through various stages, from Stage 1 to Stage 5, with Stages 3–4 accounting for nearly 70% of cases. This stage-specific data highlights the need for targeted treatments tailored to the disease’s progression.
To address both early and late-stage MSA, Alterity Therapeutics is bringing a novel molecule to the market. ATH434, a promising disease-modifying therapy (DMT) in development, is designed to address both early and late-stage MSA, aligning with this need.
The hallmark of MSA is the abnormal accumulation of alpha-synuclein in oligodendrocytes, which is thought to drive widespread cell loss in the brain. Despite this critical insight, the market still lacks an approved treatment for MSA. While significant progress has been made in experimental therapies targeting alpha-synuclein aggregation, these therapies are yet to translate into a widely accpeted solution. This raises the question:
Why has an effective alpha-synuclein-targeting drug not yet reached the market? Is it the complexity of the disease’s pathology, the challenges in clinical trials, or are there other unforeseen barriers preventing approval?
Current Therapeutic Approaches to Autonomic Dysfunction in Multiple System Atrophy (MSA)
In the ongoing battle against MSA, the current market is brimming with off-label therapies and generic drugs, none of which are authorized specifically for the condition. This glaring gap underscores the pressing need for a therapy capable of effectively managing or halting the disease’s progression.
Symptomatic management remains the cornerstone of MSA care, as no disease-modifying medication has proven effective in halting its progression. Managing MSA involves addressing a range of debilitating symptoms through tailored treatments. For parkinsonism, first-line therapy includes levodopa combined with domperidone and physiotherapy, while alternatives like dopamine agonists or amantadine may be considered. Cerebellar ataxia primarily relies on physiotherapy, supplemented by medications such as clonazepam or gabapentin. Nonpharmacological measures and adrenergic receptor agonists like midodrine are standard for orthostatic hypotension, with alternatives like fludrocortisone or droxidopa for severe cases.
According to DelveInsight’s forecast model, in 2023, adrenergic receptor agonists generated the highest revenue of approximately USD 30 million in 7MM. Furthermore, the multiple system atrophy treatment market is expected to increase at a significant CAGR by 2034 due to the expected entry of emerging molecules.
However, to date, no treatment exists that can stop or reverse the course of MSA. Nevertheless, over the past decade, research in preclinical models and patient has advanced the understanding of the disease’s underlying pathophysiological mechanisms. While the precise cause of MSA remains unclear, it is believed to be multifactorial, involving a combination of triggers that may serve as potential targets for novel therapies.
Reliance on Levodopa
“MSA is not as benign a disease as Parkinson’s disease, and it does not respond to the same treatments. It is essential to diagnose early.
– Chief Executive Officer and Cofounder, Amprion
For more than a decade, the market has heavily relied on levodopa for the treatment of MSA-P, reflecting a heavy dependence on this decades-old therapy. Despite its limited and often transient effectiveness in MSA compared to Parkinson’s disease, levodopa is widely prescribed. Its efficacy in alleviating bradykinesia and rigidity provides temporary relief, but as MSA progresses rapidly, many patients experience diminishing benefits.
Our analyst’s findings, corroborated by key opinion leaders, revealed that levodopa remains the most prescribed drug for MSA patients, accounting for nearly 30% of the total multiple system atrophy treatment market size in the 7MM in 2023. This highlights the ongoing reliance on this treatment due to the absence of disease-modifying options.
The increasing demand for innovative treatments underscores the pressing need to develop targeted strategies beyond levodopa for holistic management of MSA.
“Severely debilitating illnesses lacking treatments offer opportunities for new entrants addressing the disease’s root cause.”
– Neurologist, University of California, Irvine
A New Era of Alpha-Synuclein in Multiple System Atrophy Treatment
Alpha-synuclein, a protein primarily found in presynaptic terminals, has emerged as a pivotal target in the search for disease-modifying treatments (DMTs) for MSA. While its normal function involves neurotransmitter release and synaptic plasticity, its misfolding, aggregation, and prion-like spread across the brain are central to the progression of synucleinopathies. Recent research has shown that alpha-synuclein oligomers, rather than large fibrillar aggregates, are the primary neurotoxic species, driving neuronal dysfunction and damage.
This discovery has opened the door to innovative therapeutic strategies for MSA treatment aimed at preventing or reversing alpha-synuclein aggregation. Some promising developments in this realm include Amlenetug (currently in Phase III), TAK-341/MEDI1341 (Phase II), Emrusolmin (Phase II), and ATH434 (Phase II). Notably, Amlenetug has demonstrated its ability to inhibit the seeding of alpha-synuclein by binding on these aggregates. Additionally, the promising preclinical results of Emrusolmin further strengthen the case for alpha-synuclein inhibitors as a potential therapeutic avenue.
These findings highlight the potential of targeting alpha-synuclein as a key therapeutic pathway, offering hope for novel treatments that could modify the disease course in MSA patients and alleviate their symptoms.
Bridging the Gap Between Symptom Control and Disease Progression
Amlenetug (Lu AF82422), the anticipated first disease-modifying treatment (DMT) for MSA according to the Lundbeck’s latest reports, is a monoclonal antibody targeting alpha-synuclein. It has garnered attention from the US FDA, EMA, and Japanese health authorities. This multiple system atrophy treatment drug has received several designations enhancing its clinical pathway for approval, including Orphan Drug Designation (ODD) from both the US FDA and EMA, along with SAKIGAKE designation in Japan, further boosting its profile in the fight against MSA.
The encouraging findings from the Phase II trial showed a 19% reduction in clinical progression (measured by the UMSARS total score), but the results were not statistically significant. Nevertheless, deeper analysis revealed more consistent, promising reductions in disease progression across subscales, highlighting a potential therapeutic pathway. As the AMULET study progresses, it offers hope for more targeted therapies for MSA, marking a critical step in addressing the unmet needs of this devastating condition.
Theravance Biopharma’s Phase III molecule is also in the race as a symptomatic MSA treatment. Ampreloxetine, an investigational norepinephrine reuptake inhibitor, is being developed to treat symptomatic neurogenic orthostatic hypotension in patients with MSA. The drug functions by binding to norepinephrine transporters, thereby increasing extracellular norepinephrine levels. The Orphan Drug Designation (ODD) granted by the US FDA underscores its potential for addressing this rare condition.
Although Ampreloxetine faced a setback when its Phase III, REDWOOD trial was terminated based on efficacy results, the company remains optimistic and has initiated another Phase III trial, called CYPRESS, following discussion with the FDA. Topline results are expected by the end of 2025. If approved, Ampreloxetine could become the only durable and convenient treatment, competing with existing options such as droxidopa and midodrine.
Similarly, the interim 6-month data for ATH434-202 showed that 43% of participants improved on the UMSARS Activities of Daily Living scale. Additionally, 29% had stable or improved neurological symptoms. Objective biomarkers also demonstrated improvement, consistent with clinical findings. The topline data for ATH434-201, along with the final 12-month results from ATH434-202, is anticipated in the first half of 2025. These developments signal significant progress in targeting stage-specific MSA treatment pathways.
Future Prospects
The future of the MSA market is poised for transformation, driven by the increasing recognition of disease-modifying therapies and a focus on innovative treatment modalities. Research into the pathophysiology of MSA, especially the role of alpha-synuclein aggregation, is opening up new therapeutic avenues.
The dynamic pipeline, with numerous competitors developing innovative treatments, signals a shift towards more targeted, disease-modifying approaches. Several companies are advancing novel drug candidates, such as Amlenetug (Lu af82422), Ampreloxetine, TAK-341/MEDI1341, Emrusolmin (TEV-56286), and ATH434, among other candidates which have the potential to address unmet needs. Industry leaders like H. Lundbeck, Genmab, Theravance Biopharma, AstraZeneca, Takeda Pharma, Teva Pharmaceutical, MODAG GmbH, and Alterity Therapeutics, among others, are poised to lead the market. As these treatments advance, the market is expected to experience substantial growth, with emerging therapies likely to increase market value significantly by 2034. The future lies in precision medicine and therapies tailored to MSA’s complex mechanisms.
Among the leading multiple system atrophy treatment drug candidates in development, Amlenetug is projected to capture the highest market share by 2034, as it is anticipated to be the first entrant in the market. This success is largely attributed to its strong safety and efficacy profile.
In addition, several promising novel molecules are in mid-stage development. ATH434, an alpha-synuclein inhibitor, is expected to secure approximately 30% of the total MSA market across the 7 major markets (United States, Germany, France, Italy, Spain, the United Kingdom, and Japan), by 2034.
Furthermore, early identification of MSA using biofluid and neuroimaging biomarkers is critical, as patterns of iron accumulation differ markedly from Parkinson’s disease. However, challenges persist in defining ‘early’ MSA, which remains a somewhat fluid concept. Biomarkers such as neurofilament light are gaining prominence in improving diagnostic accuracy and refining patient selection for clinical trials.
Insights from ongoing assessments, like those involving ATH434, underscore the potential of these markers to detect iron accumulation in specific brain regions, advancing both early diagnosis and the development of targeted therapeutic approaches. These advancements aim to enhance the precision and efficacy of MSA clinical research and management strategies. This analysis is further supported by the key leaders who have shown a blend of excitement and measured optimism regarding MSA.
“A lot of times patients come in with a Parkinson’s disease diagnosis, they may have a couple of red flags that make you think, ‘Oh, this is not like a run-of-the-mill patient with Parkinson, and that might or might not be MSA…’ What we really are trying to do is come up with biofluid and neuroimaging biomarkers that help us get a better sense if this patient really has what we think is an MSA diagnosis.”
– Director, Huntington’s Disease Clinic, Vanderbilt University
A comprehensive assessment of emerging drug assets in the pipeline for MSA treatment, along with detailed analyses, is available in the final report provided by DelveInsight. This report covers anticipated drug candidates and projected launch timelines and provides in-depth analytical insights into each asset’s potential impact on the market.