10 Game-Changing Acute Myeloid Leukemia Drugs Revolutionizing Treatment
Feb 07, 2025
Table of Contents
Acute Myeloid Leukemia (AML) is a fast-moving and aggressive cancer that demands timely and effective treatment. With the current acute myeloid leukemia therapies showing limited success for many patients, the focus has shifted to breakthrough AML drugs that are redefining the treatment landscape. From innovative small-molecule inhibitors to game-changing immunotherapies, these AML drugs have the potential to revolutionize how we approach this challenging disease. In this blog, we highlight the top AML therapies to watch, offering renewed hope for those battling AML and pushing the boundaries of what’s possible in modern treatment.
What are the Current Treatment Options for Acute Myeloid Leukemia?
Several FDA-approved AML drugs, including VYXEOS (Jazz Pharmaceuticals), RYDAPT (Novartis), ONUREG (BMS), DAURISMO (Pfizer), TIBSOVO (Agios Pharma), VENCLEXTA (Abbvie), offer treatment options based on specific genetic mutations. The AML treatment landscape is continuously evolving with new drug approvals and designations. On January 22, 2025, Medexus received FDA approval for GRAFAPEX, an alkylating agent used with fludarabine for alloHSCT preparation in AML and MDS patients. Despite these advancements, AML remains a challenging disease with a high relapse rate, underscoring the need for continued research into more effective and targeted therapies. While chemotherapy and HSCT are still the standard of care, emerging treatments like targeted inhibitors, immunotherapies, and novel combination regimens are shaping the future of AML management.
Downloads
Article in PDF
Recent Articles
- AstraZeneca’s Imfinzi Shows Positive Results; Novartis Announces Results of Tislelizumab; FDA Gra...
- Daiichi Sankyo’s Ezharmia; Pfizer & Sangamo Hemophilia A Gene Therapy Trial; Approval for Fe...
- FDA Grants Fast Track for Lin BioScience’s LBS-007; Alnylam’s AMVUTTRA sNDA Under Review; FDA App...
- South Korean researchers influencing lift of human-embryo restrictions
- AbbVie Receives Approval; Kymriah Approved; Shire Gets USFDA Approval; NICE Rejects; Bristol-Myer...

Inside the Acute Myeloid Leukemia Pipeline: Key Innovations and Emerging Therapies
In recent years, the acute myeloid leukemia pipeline has witnessed an influx of innovative therapies, including small molecules, targeted therapies, and immune-based treatments. Some of these new therapies target specific genetic mutations, such as FLT3, IDH, and KMT2A, commonly found in AML patients. Others focus on enhancing the body’s immune response to leukemia cells or modifying them through gene editing technologies. These therapies offer the potential to improve remission rates and extend survival, particularly for patients with limited options with existing treatments. As the understanding of AML biology continues to deepen, researchers uncover new drug classes and innovative mechanisms of action that could transform treatment paradigms for AML patients.
In the AML treatment landscape, numerous companies are pushing the envelope in drug development. Key AML drugs to watch in the AML pipeline include Crenolanib (Arog Pharmaceuticals), Uproleselan (GlycoMimetics), Iomab-B (Actinium Pharmaceutical), BST-236 (BioSight), SLS009 (SELLAS Life Sciences), Bexmarilimab (Faron Pharmaceuticals), Ziftomenib (Kura Oncology), MK-0482 (Merck Sharp & Dohme LLC), and others. Other drugs like ImCheck Therapeutics’ ICT01, targeting immune checkpoints, are also drawing attention as they combine immunotherapy with traditional AML treatment regimens, representing an exciting evolution of treatment paradigms. Let’s take a closer look at some of the developments surrounding these AML drugs.
Arog Pharmaceuticals’ Crenolanib
Phase – III
Arog Pharmaceuticals is developing Crenolanib, an investigational small-molecule drug candidate. It is a potent inhibitor targeting both wild-type and mutant forms of FLT3 (FMS-like Tyrosine Kinase 3) and PDGFRα/β (Platelet-Derived Growth Factor Receptor). The drug has received an Orphan Drug Designation (ODD) and Fast Track Designation (FTD) from the US FDA. Currently, crenolanib is being evaluated in two Phase III clinical trials, in combination with chemotherapy, for patients with newly diagnosed acute myeloid leukemia(NCT03258931) and relapsed/refractory AML (NCT03250338).
Findings from a completed Phase II study in newly diagnosed AML patients with FLT3 mutations were presented at the ASCO 2024 Annual Meeting. The study demonstrated that crenolanib, when combined with intensive chemotherapy in adults with FLT3-mutant AML, achieved a high rate of deep responses and long-term survival with an acceptable toxicity profile.
Actinium Pharmaceutical’s Iomab-B
Phase – III
Iomab-B is a first-in-class targeted radiotherapy developed by Actinium Pharmaceuticals to improve access to bone marrow transplantation (BMT) for patients with relapsed or refractory acute myeloid leukemia (r/r AML). By targeting CD45, a protein expressed on blood cancer cells, immune cells, and bone marrow stem cells, Iomab-B helps deplete these cells rapidly, making it easier to proceed with BMT. The therapeutic has been shown to increase survival, enhance BMT access, and improve tolerability compared to traditional treatments. In the Phase III SIERRA trial, 100% of patients receiving the therapeutic dose of Iomab-B were able to access BMT, with all 66 patients undergoing successful engraftment. This contrasts sharply with the control group, where only 17% were able to access BMT as per protocol.
In addition to improving access to BMT, Iomab-B also significantly reduced the time to transplant. Patients in the Iomab-B arm reached BMT in a median of just 29 days, compared to 66.5 days for those in the control arm, who needed to achieve a complete remission before proceeding with BMT. The Journal of Clinical Oncology published the Phase III SIERRA trial results in September 2024, highlighting Iomab-B’s potential as a transformative treatment for AML patients ineligible for BMT due to refractory disease. With promising results in over 400 patients, Iomab-B could offer a significant new option for a population that represents approximately 50% of AML patients and who currently have limited treatment options.
GlycoMimetics’ Uproleselan
Phase – III
GlycoMimetics is developing uproleselan, a novel E-selectin antagonist designed for use in combination with chemotherapy to treat Acute Myeloid Leukemia and potentially other hematologic malignancies. Uproleselan specifically binds to E-selectin, a protein expressed on the surface of blood vessels, to disrupt its role in AML progression.
E-selectin binding to myeloid cells triggers pro-survival mechanisms through NF-kB signaling, providing a protective niche for leukemic cells. Uproleselan is engineered to counteract this by displacing AML cells from their survival niche, inhibiting cellular communication signals that enhance survival, and sensitizing cancer cells to chemotherapy’s cytotoxic effects.
The drug offers a promising strategy to overcome established mechanisms of leukemic cell resistance. Uproleselan is currently in Phase III clinical trials for the treatment of AML, reflecting its potential to improve outcomes in this challenging disease.
BioSight’s BST-236
Phase – II
Aspacytarabine (BST-236) is a novel proprietary anti-metabolite designed to enhance the therapeutic profile of cytarabine. It is composed of cytarabine covalently bound to asparagine, functioning as a prodrug of cytarabine. This unique composition allows the delivery of high doses of cytarabine to leukemia patients while reducing systemic exposure to the free drug, potentially minimizing associated toxicities.
Currently, aspacytarabine is in the Phase II stage of development for the treatment of Acute Myeloid Leukemia (AML), offering a promising therapeutic option for this challenging condition.
Kura Oncology’s Ziftomenib
Phase – II
Ziftomenib is a novel, oral, once-daily investigational drug candidate designed to target the menin-KMT2A/MLL protein-protein interaction, specifically addressing the needs of genetically defined AML patients with high unmet clinical needs.
In preclinical models, ziftomenib effectively inhibits the KMT2A/MLL protein complex, leading to downstream effects on HOXA9 and MEIS1 expression, which are key drivers of leukemogenesis. These actions result in potent anti-leukemic activity in genetically defined AML preclinical models.
In April 2024, The FDA granted Ziftomenib an Orphan Drug Designation for the treatment of AML. In January 2025, Kura Oncology, Inc. and Kyowa Kirin Co., Ltd. announced positive topline results from the KOMET-001 Phase II trial of ziftomenib, a selective oral menin inhibitor, in relapsed/refractory NPM1-mutant acute myeloid leukemia. Kura will present the data at a medical conference in Q2 2025 and plans to submit a New Drug Application (NDA) to the FDA in the same quarter.
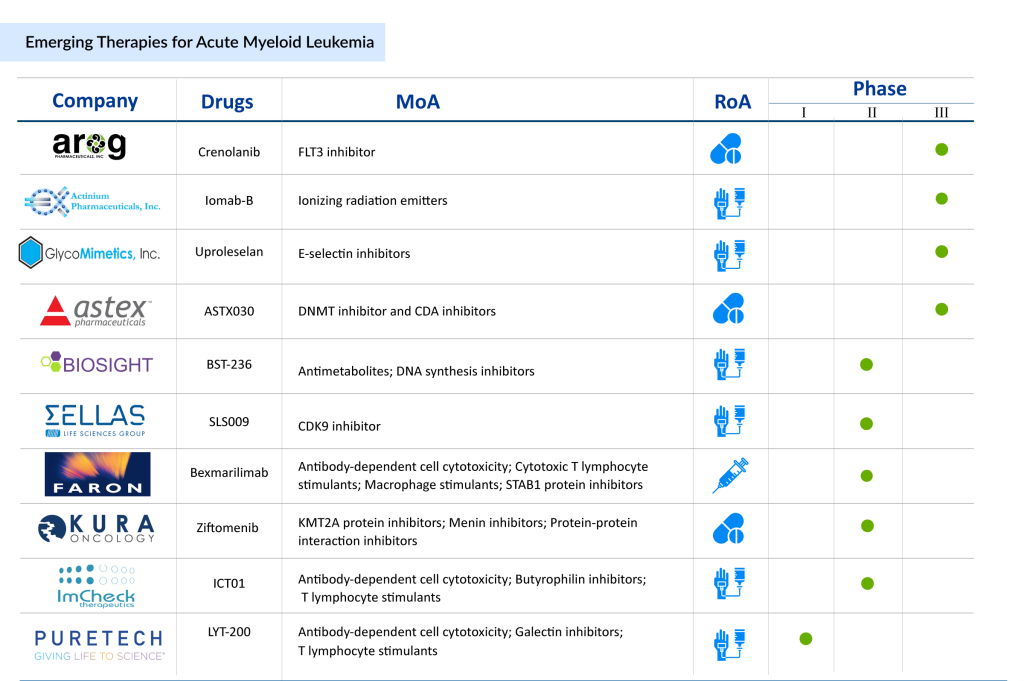
Astex Pharmaceutical’s ASTX030
Phase – II/III
ASTX030 is an investigational oral combination therapy of cedazuridine and azacitidine, developed to provide an effective and convenient alternative to subcutaneous (SC) azacitidine. Cedazuridine enhances azacitidine’s bioavailability by inhibiting its breakdown in the gut, ensuring consistent drug levels with oral administration. This approach aims to simplify treatment administration while maintaining efficacy for patients with Myelodysplastic Syndromes (MDS), Chronic Myelomonocytic Leukemia (CMML), and Acute Myeloid Leukemia.
The ASTX030-01 study is a multi-phase clinical trial evaluating the safety, efficacy, and pharmacokinetics of ASTX030. The study follows a structured progression, beginning with Phase I, which includes a Dose Escalation Stage (Stage A) followed by a Dose Expansion Stage (Stage B). Phase II is a randomized, open-label crossover study comparing oral ASTX030 to SC azacitidine, while Phase III further evaluates the final oral ASTX030 dose against SC azacitidine. The trial is expected to last approximately 48 months, with completion anticipated in April 2026.
SELLAS Life Sciences’ SLS009
Phase – I/II
SLS009 is a highly selective, first- and best-in-class small molecule CDK9 inhibitor with a differentiated profile. Compared to other CDK9 inhibitors, SLS009 demonstrates reduced toxicity and enhanced potency. In patients with acute myeloid leukemia (AML), data has highlighted its high response rate, particularly in those with unfavorable prognostic factors such as the ASXL1 mutation.
In July 2024, the FDA granted SLS009 Rare Pediatric Disease Designation (RPDD) for the treatment of pediatric AML. Previously, it received Orphan Drug Designation (ODD) for R/R AML, and in January 2024, Fast Track Designation for the same indication.
SLS009 is currently under evaluation in a Phase I/II clinical trial involving patients with hematologic malignancies, including relapsed/refractory AML. In March 2024, the company announced positive topline results from the Phase IIa portion of the study. When combined with azacitidine and venetoclax, SLS009 achieved an overall response rate (ORR) of 50% in relapsed/refractory AML patients treated at the optimal dose of 30 mg twice weekly—surpassing the target response rate of 20%.
Faron Pharmaceuticals’ Bexmarilimab
Phase – I/II
Bexmarilimab, developed by Faron Pharmaceuticals, is a first-in-class humanized antibody that targets Clever-1 on myeloid cells and macrophages to enhance anti-tumor immunity. This innovative mechanism aims to modulate immune responses, offering a novel therapeutic approach.
In January 2024, Faron initiated Phase II of the BEXMAB trial, dosing the first patient to evaluate the safety and efficacy of bexmarilimab in combination with the standard of care (SoC). The trial focuses on patients with hypomethylating agents (HMAs)-refractory or relapsed myelodysplastic syndrome (MDS), a highly aggressive myeloid leukemia with limited treatment options.
ImCheck Therapeutics’ ICT01
Phase – I/II
ICT01 is a first-in-class, Fc-disabled anti-BTN3A monoclonal antibody that selectively activates γ9δ2 T cells, driving direct cytotoxicity against AML blasts and enhancing immune response through CD8 T cells and NK cells. It enhances the anti-leukemic effect of venetoclax (V), which overcomes granzyme B/perforin resistance in AML blasts, and azacitidine (A), which improves immune cell recognition of AML. Preclinical studies showed that ICT01 protects γ9δ2 T cells and NK cells from venetoclax-induced cell death, highlighting its therapeutic potential.
In September 2024, the FDA granted Fast Track Designation to ICT01 in combination with azacitidine and venetoclax for AML patients aged 75 or older, or those unable to tolerate standard chemotherapy. This designation reflects the urgent need for alternative treatments. Based on positive results from the Phase 1 EVICTION study evaluating ICT01 monotherapy in relapsed/refractory hematological malignancies (European Society for Medical Oncology Congress 2023), ImCheck initiated a randomized dose-optimization cohort in October 2023, testing two doses of ICT01 with azacitidine and venetoclax for newly diagnosed AML patients unfit for induction chemotherapy.
PureTech Health’s LYT-200
Phase – I
LYT-200 is in development as a potential treatment for hematologic malignancies like AML and high-risk MDS, as well as locally advanced or metastatic solid tumors, including head and neck cancers. This fully human IgG4 monoclonal antibody targets galectin-9, a potent oncogenic driver in leukemia cells and an immunosuppressive protein expressed by both tumors and immune cells. Galectin-9 has been shown to inhibit the immune system’s ability to recognize and eliminate cancer cells. Extensive preclinical data highlight the therapeutic potential of LYT-200, underscoring the significance of galectin-9 as a target and pointing to the opportunity for biomarker development.
In January 2025, LYT-200 received Fast Track Designation from the FDA, emphasizing the urgent need for new treatment options in AML. Regulators grant this designation to therapies that address serious conditions and meet significant unmet medical needs, expediting their development and review. The Fast Track status underscores the potential of LYT-200 to make a meaningful impact in the treatment of AML and other challenging cancers.
The Road Ahead for AML Treatment
The AML pipeline is teeming with innovations that have the potential to revolutionize the treatment of acute myeloid leukemia. From novel small-molecule inhibitors targeting genetic mutations to cutting-edge immunotherapies and epigenetic modulators, the development of AML therapies is advancing rapidly. Promising treatments like Revumenib, Crenolanib, and SLS009 are showing early success in clinical trials, offering new hope for patients previously limited by treatment options. Alongside drug advancements, technologies such as gene editing, CRISPR gene therapy, and CAR-T cell therapies are also being explored. These promising techniques target leukemia stem cells resistant to traditional treatments, significantly improving remission rates and potentially reducing the risk of relapse.
The increasing shift toward precision medicine is further reshaping AML treatment strategies, focusing on personalized therapies tailored to individual patient profiles. Combination therapies, in particular, are gaining traction, improving remission rates and extending life expectancy for high-risk patients. The focus on these targeted approaches brings optimism to a disease long associated with poor outcomes, offering fresh hope for many patients who previously had limited treatment options. These innovations aim to enhance survival and improve patients’ quality of life, turning the tide in the fight against this aggressive disease.
Despite these breakthroughs, challenges remain in developing and commercializing new AML therapies. The complexity of the disease and the need for more effective, safer treatments highlight the importance of continued innovation and rigorous clinical testing. While regulatory hurdles and high costs present significant obstacles, initiatives like Fast Track Designations for promising AML drugs such as LYT-200 and ICT01 provide hope that these treatments will reach patients faster. With ongoing research uncovering new therapeutic targets, the future of AML treatment looks promising, with the potential to offer better options and improved outcomes for patients battling this challenging disease.

Downloads
Article in PDF
Recent Articles
- Transforming Multiple Myeloma Treatment: The Promise of Novel Drug Classes
- Gene Therapy’s Emergence: The “New” approach for Huntington’s disease
- Abingworth & Alebund’s Finacial Closing; Pfizer/BioNTech COVID-19 Vaccine Expanded Use...
- DelveInsight’s Cardiovascular disorders based Gene Therapy Reports
- Iovance’s Breakthrough: Amtagvi Paves the Way for Cell Therapies in Solid Tumors