Acute Myeloid Leukemia: The Battle, The Breakthroughs, The Future
Feb 10, 2025
Table of Contents
Acute Myeloid Leukemia (AML) is a rapidly progressing cancer that originates in the bone marrow—the soft, inner part of bones where blood cells are produced—and swiftly moves into the blood. Globally, the burden of AML has been increasing over the past few decades. The exact cause of AML remains elusive, but several risk factors have been identified. Age plays a significant role, with the disease being more prevalent in individuals aged 65 and older. Prior cancer treatments, especially certain types of chemotherapy and radiation therapy, can increase the risk. Exposure to high levels of radiation, such as from nuclear reactor accidents, and contact with hazardous chemicals like benzene are also linked to a heightened risk of developing AML.
Acute myeloid leukemia symptoms can vary but often include fatigue, frequent infections, easy bruising or bleeding, and unexplained weight loss. Some individuals may experience bone pain or notice small red spots on the skin due to bleeding under the skin. These symptoms arise because the proliferation of abnormal white blood cells in the bone marrow interferes with the production of normal blood cells, leading to anemia, susceptibility to infections, and impaired blood clotting.
Downloads
Article in PDF
Recent Articles
- 6 Emerging Treg Cell-based Therapies Shaping the Future of Immunotherapy
- Predicting risk of Acute Myeloid Leukemia (AML) in healthy individuals
- FDA Grants Priority Review for Zolbetuximab BLA; FDA Traditional Approval for LEQEMBI for Alzheim...
- Antibody–Drug Conjugates: An Emerging Concept in Cancer Therapy
- Venclexta full approval; PBP1510 Orphan Status; Roche/ Genesis collab; Immunomodulators for COVID-19
Diagnosing AML typically involves a combination of blood tests and bone marrow examinations. A complete blood count can reveal abnormalities in blood cell levels, prompting further investigation. A definitive diagnosis is usually confirmed through a bone marrow biopsy, where a sample is examined for the presence of leukemic cells. Additional tests, such as genetic studies, can provide insights into specific mutations that may influence treatment decisions and prognosis.
Understanding AML is crucial for early detection and effective treatment. As research advances, there is hope for improved therapies and outcomes for those affected by this challenging disease.
Acute Myeloid Leukemia Epidemiology and Patient Numbers
According to DelveInsight’s Acute Myeloid Leukemia Epidemiology-Based Market Report, the total incident cases of Acute Myeloid Leukemia across the 7MM in 2023 were approximately 43.5K. Among these, the United States accounted for the highest number of incident cases, with around 21.3K cases. In the EU4 (Germany, France, Italy, Spain) and the UK, Germany reported the highest number of AML cases, while Spain recorded the lowest incidence.
AML predominantly affects older adults, with the average age at diagnosis being approximately 69 years. The disease is uncommon in individuals under the age of 45, though it can occur in children as well. AML epidemiological data indicate a slight male predominance, with 52% of cases occurring in males and 48% in females.
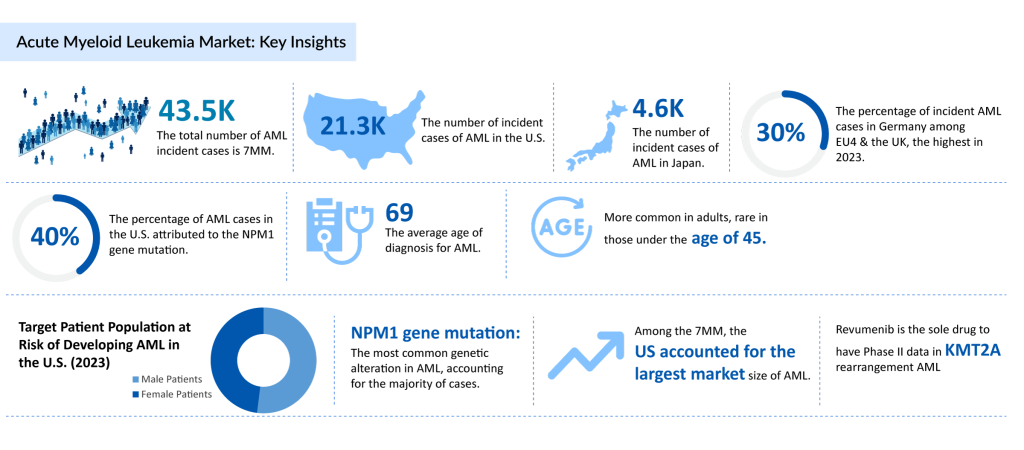
Among the various genetic mutations linked to AML, NPM1 gene mutations were observed in approximately 25–30% of newly diagnosed cases, making it one of the most frequently occurring mutations. Other notable mutations include FLT3 (~30% of cases), IDH1/2 (~20%), and TP53 (~5–10%), all of which significantly impact prognosis and treatment outcomes.
With an aging population and evolving genetic insights, the acute myeloid leukemia incidence is expected to rise, necessitating continuous advancements in treatment approaches and therapeutic strategies.
Uncover the financial impact of Acute Myeloid Leukemia and the path to impactful solutions—read our latest blog now!
Acute Myeloid Leukemia: A Cancer Without Stages?
Unlike solid tumors, Acute Myeloid Leukemia does not follow a traditional staging system because it originates in the bone marrow and spreads throughout the blood. However, AML progression can be categorized into different phases based on disease severity, response to treatment, and patient prognosis.
First Stages of Acute Myeloid Leukemia
The early stages of acute myeloid leukemia often begin with subtle symptoms such as fatigue, unexplained bruising, frequent infections, and pale skin. In this phase, abnormal myeloid cells start accumulating in the bone marrow, leading to reduced production of normal blood cells. Diagnosis at this stage is confirmed through bone marrow biopsies, blood tests, and genetic mutation analysis to determine risk stratification and treatment options.
Progression and Relapsed/Refractory AML
AML progresses rapidly, requiring immediate intervention. Patients typically undergo induction chemotherapy to achieve remission, followed by consolidation therapy or hematopoietic stem cell transplantation (HSCT) to prevent relapse. However, some cases become refractory AML, meaning the disease does not respond to initial treatment. Others may relapse, necessitating additional targeted therapies or clinical trials.
Final Stages of Acute Myeloid Leukemia
In advanced or final stages of acute myeloid leukemia, the disease becomes more aggressive, and treatment-resistant leukemic cells proliferate uncontrollably. Patients may experience severe infections, extensive bleeding, and multi-organ complications due to bone marrow failure. Supportive care, palliative treatments, and targeted therapies are often considered in these cases to improve quality of life.
Since acute myeloid leukemia stages differ from typical cancer staging, risk-based classifications (low, intermediate, and high-risk AML) help guide treatment decisions. With ongoing advancements in precision medicine, newer targeted therapies and immunotherapies are transforming AML management, offering hope for improved survival outcomes.
Treatment of Acute Myeloid Leukemia
The treatment of Acute Myeloid Leukemia typically involves a combination of chemotherapy, targeted therapy, and, in some cases, stem cell transplantation. The approach depends on multiple factors, including the patient’s age, overall health, genetic mutations, and response to initial treatment.
Standard Acute Myeloid Leukemia Treatments
Most acute myeloid leukemia treatment strategies follow a two-phase chemotherapy approach:
- Remission Induction Therapy: The goal is to eliminate as many leukemia cells as possible and achieve remission. This is usually done with intensive chemotherapy-based induction therapy, such as the “7+3 regimen” (cytarabine and an anthracycline like daunorubicin or idarubicin). However, due to its intensity, this therapy is generally recommended for younger or physically fit patients.
- Consolidation Therapy (Post-Remission Therapy): Even after achieving remission, some minimal residual disease (MRD) remains, which can lead to relapse. To reduce this risk, consolidation therapy with high-dose cytarabine (HiDAC) or hematopoietic stem cell transplantation (HSCT) is recommended for high-risk patients. Stem cell transplants are particularly effective in preventing relapse but come with significant risks, including graft-versus-host disease (GVHD) and severe infections.
For elderly or unfit patients, lower-intensity chemotherapy regimens or targeted therapies are often preferred due to the toxicity of standard induction therapy.

Targeted therapies, which focus on specific genetic mutations driving the disease, have reshaped the AML treatment landscape in recent years. Drugs such as VYXEOS (Jazz Pharmaceuticals), RYDAPT (Novartis) for FLT3-mutated AML, and VENCLEXTA (AbbVie) for newly diagnosed patients ineligible for intensive chemotherapy have provided promising outcomes. Other approved treatments include ONUREG (BMS) for maintenance therapy, TIBSOVO (Agios Pharmaceuticals) for IDH1-mutated AML, and XOSPATA (Astellas Pharma) for relapsed/refractory FLT3-mutated cases. Despite these advancements, AML remains a difficult-to-treat cancer with high relapse rates, necessitating the development of novel treatment approaches.
Market Understanding of Acute Myeloid Leukemia
The Acute Myeloid Leukemia market has witnessed significant advancements in recent years, driven by emerging therapies, targeted treatments, and ongoing research in immunotherapies and cell-based therapies. Despite the longstanding reliance on intensive chemotherapy and hematopoietic stem cell transplantation (HSCT) as the standard of care, newer targeted therapies have reshaped the treatment landscape, offering more personalized options for patients with specific genetic mutations.
Among the most widely used targeted therapies, RYDAPT (Novartis) and XOSPATA (Astellas Pharma) focus on FLT3 mutations, present in approximately 30% of AML cases. TIBSOVO (Agios Pharmaceuticals) and IDHIFA (BMS) address IDH1 and IDH2 mutations, respectively. Additionally, VENCLEXTA (AbbVie), a BCL-2 inhibitor, has gained traction for use in combination with hypomethylating agents or low-dose cytarabine in patients who are ineligible for intensive chemotherapy. These advancements reflect the market’s shift toward mutation-specific therapies, allowing for better survival outcomes and lower toxicity compared to traditional chemotherapy.
Despite these innovations, not all patients are eligible for intensive therapy, which remains physically and mentally demanding. This challenge has fueled the demand for alternative approaches, particularly for older patients or those with high-risk genetic profiles. In recent years, menin inhibitors have emerged as promising agents in AML subtypes driven by KMT2A rearrangements and NPM1 mutations. Investigational drugs such as Revumenib (Syndax Pharmaceuticals), Ziftomenib (Kura Oncology), and JNJ-75276617 (Janssen) have demonstrated complete remission rates of 20-35% in early clinical trials, making them key players in the future AML market.
The final stages of AML often shift the focus from curative treatment to palliative care, emphasizing symptom management and quality of life. Supportive treatments such as radiation therapy, blood transfusions, and opioid-based pain management (e.g., morphine) play a crucial role in easing patient discomfort. For some, low-dose chemotherapy or hypomethylating agents may help slow disease progression, but overall, the need for more effective and less toxic late-stage treatment options remains a key market opportunity.
Recent Breakthroughs in the Acute Myeloid Leukemia Treatment Landscape
The acute myeloid leukemia pipeline is constantly evolving, with ongoing clinical trials, regulatory approvals, and advancements in drug development. Every month, new therapies enter the pipeline, investigational drugs receive designations to accelerate their development, and pharmaceutical companies make strategic moves to enhance treatment options for AML patients. The past few months have been particularly eventful, with significant updates shaping the future of AML care.
- In February 2025, Auron Therapeutics, a clinical-stage biotechnology company focused on cell-state plasticity to enhance outcomes in oncology and inflammatory diseases, announced progress in its lead KAT2A/B program and clinical candidate, AUTX-703. The company also completed a $27 million Series B financing.
- In January 2025, Medexus announced that the FDA approved GRAFAPEX™, an alkylating agent, in combination with fludarabine as a preparative regimen for allogeneic hematopoietic stem cell transplantation (alloHSCT) in adult and pediatric patients aged one year and older with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS).
- In January 2025, PureTech Health revealed that the FDA granted Fast Track designation to its investigational drug LYT-200 for the treatment of AML. This designation is intended to accelerate the drug’s development and review process, recognizing the urgent need for new treatment options for AML patients.
- In October 2024, the FDA granted Fast Track designation to HC-7366, a novel GCN2 kinase modulator, for the treatment of adult patients with relapsed or refractory AML.
- In September 2024, ImCheck Therapeutics announced that the FDA granted Fast Track designation to ICT01 in combination with azacitidine and venetoclax for treating AML patients aged 75 or older or those with comorbidities that prevent them from receiving standard chemotherapy. The designation follows encouraging results from the Phase 1 EVICTION study.
With the AML market rapidly evolving, pharmaceutical and biotechnology companies continue to drive innovation, bringing forth new hope for patients. The coming months are expected to bring further breakthroughs, with novel targeted therapies, immunotherapies, and combination regimens poised to redefine AML treatment and improve patient outcomes.
Emerging Drugs in Acute Myeloid Leukemia
As the acute myeloid leukemia drugs landscape continues to evolve, novel investigational therapies are being developed to address unmet medical needs.
Crenolanib (Arog Pharmaceuticals) – Crenolanib is a small molecule inhibitor of wild-type and mutant FLT3 and PDGFRα/β, currently being evaluated in Phase III clinical trials for newly diagnosed and relapsed/refractory AML. The drug has received Orphan Drug Designation (ODD) and Fast Track Designation (FTD) from the US FDA. Results from a Phase II trial presented at ASCO 2024 highlighted its potential, showing deep responses and long-term survival with acceptable toxicity when combined with intensive chemotherapy in FLT3-mutant AML patients.
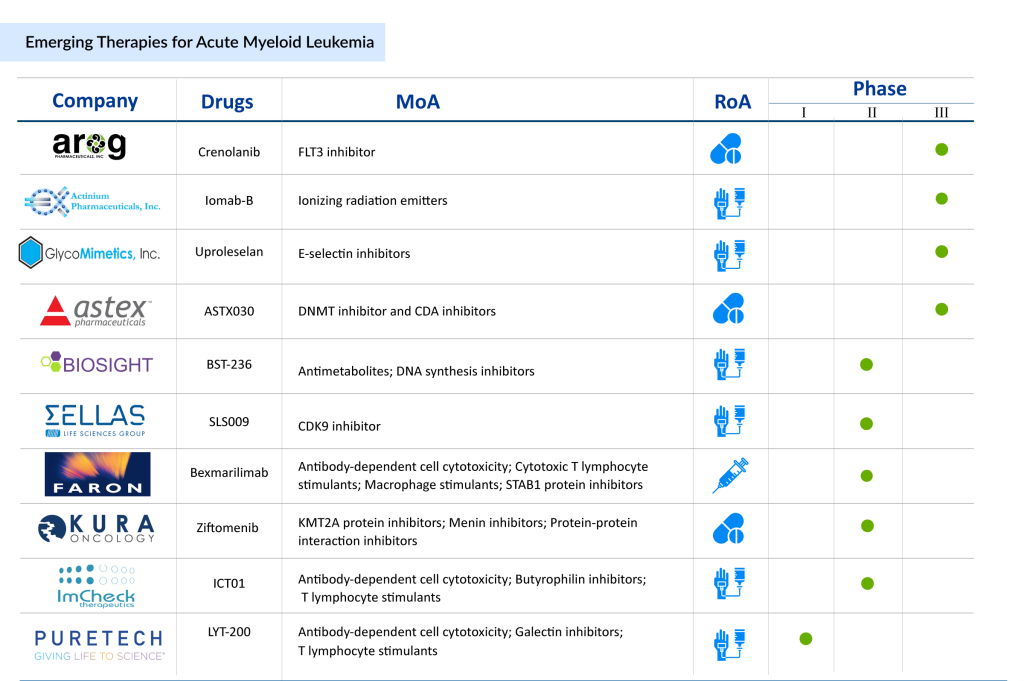
SLS009 (SELLAS Life Sciences) – SLS009 is a highly selective CDK9 inhibitor with reduced toxicity and increased potency compared to other inhibitors in its class. In AML patients, particularly those with ASXL1 mutations, the drug has demonstrated promising efficacy. The FDA granted SLS009 a Rare Pediatric Disease Designation (RPDD) in July 2024 for pediatric AML and previously provided orphan drug and fast-track designations for relapsed/refractory AML. A Phase I/II trial is currently evaluating its effectiveness in hematologic malignancies, with topline findings from the Phase IIa study revealing a 50% overall response rate (ORR) when combined with azacitidine and venetoclax, exceeding the target response rate of 20%.
Beyond these, several key players are actively shaping the AML therapeutic landscape, including Pfizer, Bristol Myers Squibb, Agios Pharmaceuticals, AbbVie, Jazz Pharmaceuticals, Astellas Pharma, Novartis Oncology, Daiichi Sankyo, AstraZeneca/Astex Pharmaceuticals, Chimerix, Takeda, Rafael Pharmaceuticals, Delta-Fly Pharma, GlycoMimetics Incorporated, and many more.
But that’s just the tip of the iceberg! discover the full pipeline of emerging therapies and see which companies are leading the market. Read our in-depth analysis now!
The Future of Acute Myeloid Leukemia Treatment
The fight against acute myeloid leukemia is relentless, but so is the progress in research, innovation, and treatment. With each new breakthrough, the outlook for patients facing this aggressive disease improves. From targeted therapies and immunotherapies to stem cell transplants and precision medicine, the treatment landscape is evolving at an unprecedented pace.
For patients and their families, one of the most pressing concerns is how long can you have acute myeloid leukemia and what factors influence acute myeloid leukemia life expectancy. While AML is known for its rapid progression, survival rates are steadily improving due to advanced diagnostics, risk-adapted therapies, and cutting-edge drug developments. The emergence of novel AML treatments and personalized approaches offers renewed hope, extending remission periods and improving quality of life.
As the market continues to evolve, the next generation of AML therapies holds the promise of more effective, less toxic, and longer-lasting treatment options. With continued advancements, collaborations, and scientific discoveries, the future of acute myeloid leukemia treatment is brighter than ever.

Downloads
Article in PDF
Recent Articles
- Precigen’s PRGN-3006; Yescarta Approved as a First CAR T-cell Therapy for R/R LBCL; Biogen ...
- AbbVie Announces Results of Study Evaluating SKYRIZI; FDA Fast Track Designation to Arrowhead’s A...
- Antibody–Drug Conjugates: An Emerging Concept in Cancer Therapy
- FDA Approves BMS’s Reblozyl for MDS; FDA Awards Orphan Drug Designation to NXC-201; Janssen Submi...
- 5 Most Promising CAR T-cell Therapies for Multiple Myeloma in Development