5 Most Promising CAR T-cell Therapies for Multiple Myeloma in Development
Mar 14, 2025
Table of Contents
The success of CAR-T therapies like Bristol-Myers Squibb/Bluebird bio’s ABECMA and Janssen’s CARVYKTI is reshaping treatment paradigms, challenging traditional options like stem cell transplants and proteasome inhibitors. The multiple myeloma market size in the US is responding rapidly from ~USD 15 billion, with increased investment and innovation driving next-generation CAR-T products with improved durability, reduced toxicity, and faster manufacturing times.
As clinical research advances, CAR-T therapies are increasingly targeting earlier lines of treatment, expanding their application beyond heavily pretreated patients. This shift is being driven by promising efficacy data in earlier-stage multiple myeloma patients, coupled with growing physician confidence in these therapies. Regulatory bodies are also accelerating review timelines for innovative CAR-T solutions, ensuring faster access for patients in need.
Downloads
Article in PDF
Recent Articles
- Acute Myeloid Leukemia: The Battle, The Breakthroughs, The Future
- Understanding High Risk Smoldering Multiple Myeloma: A Bridge Between MGUS and Multiple Myeloma
- Unleashing the Potential: CD38 Directed Therapies Revolutionize Multiple Myeloma Treatment
- Novartis’ Canakinumab for NSCLC; Novartis’s Zolgensma Updates; Trodelvy Prospects in New Breast C...
- AstraZeneca’s Imfinzi Shows Positive Results; Novartis Announces Results of Tislelizumab; FDA Gra...
5 CAR T-cell Therapies for Multiple Myeloma to Look Out For
CAR T-cell therapies are transforming the multiple myeloma landscape, offering new hope to patients with relapsed or refractory disease. By engineering a patient’s own T cells to target specific antigens like BCMA on myeloma cells, CAR-T therapies deliver a powerful and precise attack on cancer. This personalized approach has shown unprecedented response rates, even in heavily pretreated patients, with some therapies achieving deep and lasting remissions.
Key companies such as Arcellx (Anito-cel), Novartis (PHE885), Bristol-Myers Squibb (BMS-986393), CARsgen Therapeutics (Zevorcabtagene Autoleucel), and Galapagos (GLPG5301), among others, are evaluating their lead CAR-Ts for multiple myeloma treatment in different stages. Let’s dive deep into the most promising multiple myeloma drugs in development that could transform the therapeutic segment.
Arcellx’s Anito-cel (CART-ddBCMA)
Phase III
Anito-cel (anitocabtagene autoleucel) is Arcellx’s CAR-T therapy targeting BCMA, designed with the company’s proprietary BCMA-targeting binding domain to treat relapsed or refractory multiple myeloma. The therapy, also known as CART-ddBCMA, is currently in a Phase II pivotal multiple myeloma trial (iMMagine-1) for relapsed or refractory multiple myeloma and is being developed in partnership with Kite to seek regulatory approval. Arcellx’s proprietary binding domains are synthetic proteins designed to target specific therapeutic markers. The company is also evaluating this drug in Phase III confirmatory iMMagine-3 trial.
The US FDA granted Fast Track Designation (FTD) to anito-cel. In March 2020, CART-ddBCMA received Orphan Drug Designation (ODD) from the FDA for treating multiple myeloma, and in May 2021, it was awarded Regenerative Medicine Advanced Therapy (RMAT) designation for the same indication.
In December 2022, Arcellx and Kite, a Gilead Company, established a strategic partnership to co-develop and co-commercialize CART-ddBCMA for relapsed or refractory multiple myeloma. Under the agreement, Kite and Arcellx will jointly manage the development and commercialization of anito-cel in the US, while Kite will handle commercialization outside the US. In November 2023, Arcellx modified its collaboration agreement with Kite and signed a common stock purchase agreement and an amended standstill and stock restriction agreement with Gilead.
In December 2024, Arcellx reported promising new results from its Phase II pivotal iMMagine-1 multiple myeloma trial of anito-cel. The findings were shared during an oral presentation at the 66th American Society of Hematology (ASH) Annual Meeting and Exposition on Monday, December 9, 2024, at 5:30 p.m. PT.
The Phase II iMMagine-1 data, based on a cutoff date of October 31, 2024, reflect a median follow-up of 9.5 months for the efficacy evaluable group. At the cutoff, 86 patients had been evaluated for efficacy after a minimum of two months of follow-up following anito-cel treatment, while 98 patients were assessed for safety after at least one month of follow-up. All patients received a single infusion of anito-cel at a target dose of 115×10⁶ CAR+ viable T cells. Among the safety-evaluable group, 85 of 98 patients (87%) were triple-refractory, and 41 of 98 (42%) were penta-refractory. Patients had undergone a median of four prior treatment lines, with 45 of 98 patients (46%) having received three previous lines of therapy.
Novartis’ PHE885
Phase II
PHE885 is an experimental, autologous BCMA-targeting CAR-T cell therapy developed using the T-Charge platform, which showed encouraging outcomes in patients with relapsed or refractory multiple myeloma in a Phase I, first-in-human, multicenter, dose-escalation study. The T-Charge manufacturing process allows PHE885 CAR-T cells to expand rapidly within the patient’s body in under two days and maintain high levels for prolonged periods. A Phase II multiple myeloma clinical trial is currently underway to assess its effectiveness in relapsed and refractory multiple myeloma, with plans to explore its use in earlier treatment stages. These findings suggest that PHE885 has strong potential as a treatment for multiple myeloma, and ongoing research aims to further evaluate its efficacy in different settings.
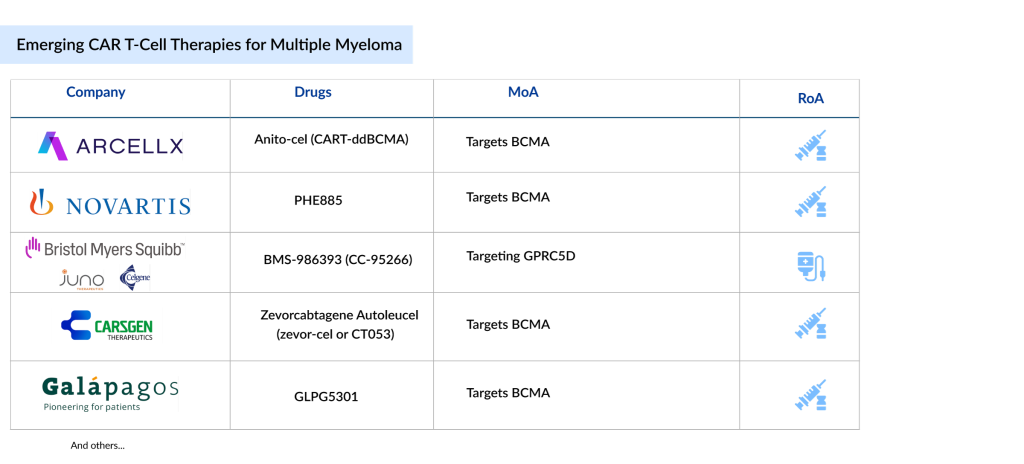
Bristol-Myers Squibb’s BMS-986393 (CC-95266)
Phase II
BMS-986393 is a pioneering autologous CAR T cell therapy targeting GPRC5D, with the potential to become a leading treatment option for its target population. It is currently being evaluated in a Phase II trial for relapsed or refractory multiple myeloma (RRMM).
In December 2024, at ASH 2024, BMS presented the first overall survival (OS) and progression-free survival (PFS) data for arlo-cel during an oral presentation. The Phase I study included patients with relapsed/refractory multiple myeloma who had previously received at least three therapies, including a proteasome inhibitor, an immunomodulatory agent, and anti-CD38 therapy. After a median follow-up of 16.1 months (range: 2.8–25.2) in 79 efficacy-evaluable patients, arlo-cel demonstrated durable responses with an overall response rate (ORR) of 87%. Minimal residual disease (MRD) was assessed as an exploratory endpoint, with 57% (48 of 84) of MRD-evaluable patients included. Of these, 46% (22 of 48) were MRD-negative and achieved a complete response (CR) or stringent CR (sCR). Across all treated patients, 27% (23 of 84) were MRD-negative and achieved a CR. Median PFS was 18.3 months (95% CI: 11.8–21.9), while median OS was not reached.
The most common treatment-related adverse events (TRAEs) were hematological, with neutropenia reported in 62 patients (74%). Cytokine release syndrome (CRS) occurred in 69 patients (82%). Three patients developed macrophage activation syndrome/hemophagocytic lymphohistiocytosis, and eight experienced immune effector cell-associated neurotoxicity syndrome (ICANS). Safety data showed that off-tumor adverse events were infrequent, mild, and typically self-limiting.
These findings support arlo-cel’s potential as a first-in-class treatment for heavily pretreated RRMM and justify its continued evaluation in the ongoing Phase II QUINTESSENTIAL study.
CARsgen Therapeutics’ Zevorcabtagene Autoleucel (zevor-cel or CT053)
Phase I/II
Zevorcabtagene autoleucel (zevor-cel or CT053) is an autologous CAR T-cell therapy designed with a fully human BCMA-specific single-chain variable fragment (25C2) that exhibits high binding affinity and a high monomer ratio. In February 2024, the NMPA approved zevor-cel for treating adults with relapsed or refractory multiple myeloma who have previously undergone at least three lines of therapy, including at least one proteasome inhibitor and one immunomodulator.
CARsgen is currently conducting the Phase Ib/II LUMMICAR STUDY 2 clinical trial in North America to assess the safety and effectiveness of zevor-cel in RRMM. According to the company, they plan to submit a Biologics License Application (BLA) in the US in the first half of 2025 based on the pivotal LUMMICAR STUDY 2 results.
Zevor-cel has received several regulatory designations: RMAT from the FDA for RRMM in October 2019, PRIME status from the EMA for RRMM in September 2019, and Breakthrough Therapy Designation (BTD) from the NMPA in December 2020. Additionally, it was granted Orphan Drug Designation (ODD) from the US FDA in 2019 and Orphan Medicinal Product Designation (OMPD) from the EMA in 2020. The NMPA also awarded zevor-cel priority review status in October 2022.
Galapagos’ GLPG5301
Phase I/II
GLPG5301 is an autologous, second-generation CAR-T therapy targeting BCMA through a 4-1BB co-stimulatory domain. It is administered as a single fixed-dose intravenous infusion of a fresh product at the point of care. The therapy is currently being evaluated in the PAPILIO-1 Phase I/II trial.
PAPILIO-1 is an open-label, multi-center Phase I/II study designed to assess the feasibility, safety, and effectiveness of point-of-care manufactured GLPG5301 in patients with relapsed or refractory multiple myeloma (rrMM) who have undergone at least two prior lines of therapy. The main goal of Phase 1 is to evaluate the safety and establish the recommended dose for Phase II. The primary objective of Phase 2 is to measure the efficacy of GLPG5301 based on the objective response rate (ORR). Secondary objectives across both phases include further safety evaluation, additional efficacy measures such as minimal residual disease (MRD) status, and assessing the feasibility of manufacturing GLPG5301 at the point of care for rrMM patients. Each patient will be monitored for 24 months.
In Phase I, up to three dose levels will be tested, with a minimum of 12 patients enrolled to identify the recommended Phase II dose. Phase II will enroll approximately 30 more patients to confirm the safety and efficacy of GLPG5301.
Future Outlook of CAR T-cell Therapies for Multiple Myeloma
The future of CAR T-cell therapies for multiple myeloma looks incredibly promising, with rapid advancements in research and clinical outcomes signaling a new era in treatment. Innovations in CAR T-cell design, including dual-targeting constructs and enhanced persistence strategies, are improving response rates and durability. Next-generation therapies are focusing on reducing relapse rates by targeting antigens like BCMA more effectively and combining CAR-T with immunomodulators to boost efficacy. With more allogeneic (off-the-shelf) CAR T therapies in the pipeline, accessibility and scalability are set to improve, potentially making these life-saving treatments more widely available.
Emerging strategies are also tackling key challenges like treatment resistance and toxicity. Researchers are exploring armoring CAR T-cells with cytokine support and gene editing to enhance their survival and potency within the tumor microenvironment. Personalized approaches, where CAR T-cells are tailored to the patient’s specific tumor profile, are also on the horizon, promising more precise and effective treatment.
Besides IMiDs, CAR-Ts, and bispecific antibodies, the multiple myeloma pipeline also includes small molecules, ADCs, and interferons. Within the BCMA bispecific class, Regeneron Pharmaceuticals’ linvoseltamab is emerging with a differentiated and compelling clinical profile in refractory multiple myeloma.
As regulatory approvals expand and manufacturing processes become more streamlined, these therapies are poised to redefine the multiple myeloma treatment landscape, offering renewed hope for long-term remission and improved patient outcomes.

Downloads
Article in PDF
Recent Articles
- Novartis’ Canakinumab for NSCLC; Novartis’s Zolgensma Updates; Trodelvy Prospects in New Breast C...
- Wave Life Sciences stocks implode; Amarin’s VASCEPA approval; GSK seeks approval
- FDA Approves BMS’s Reblozyl for MDS; FDA Awards Orphan Drug Designation to NXC-201; Janssen Submi...
- Kiadis inks license deal with Sanofi; Gilead Ends Hep B Collaboration; Merck & Foghorn inks ...
- Rising of Orphan Drug Development