AI-Driven Diagnostics: Why are They the Next Big Thing in Healthcare?
Apr 09, 2025
Table of Contents
Just a few years back, imagining a machine detecting a heart attack before symptoms surfaced or spotting a tumor more accurately than the human eye sounded like science fiction. Today, it’s just another day in AI-powered healthcare diagnostics.
Artificial Intelligence is no longer a distant promise—it’s a living, breathing part of clinical workflows, diagnostics, treatment planning, and patient engagement. From decoding complex ECG patterns to flagging sepsis in real time and revolutionizing radiology with faster, smarter image analysis, AI is quietly becoming the most trusted assistant in the room.
Downloads
Article in PDF
Recent Articles
- Merck’s Remicade; Sanofi, Regeneron set Kevzara; Novartis to sever; Teva puts women’s...
- FDA Approves; AZ nabs; Nordisk settles; Novartis pays
- FDA Approval to DePuy TELIGEN System; FDA Breakthrough Device Designation for the EndoStim System...
- BGB-16673 Breakthrough: A Tolerable Triumph in Rapid Clinical Responses for Relapsed/Refractory B...
- Liso-cel Shines in TRANSCEND FL: Impressive Complete Responses, Durable Overall Responses, and Ma...
Its strength lies in speed, scale, and precision. With the ability to sift through oceans of data—far beyond human capability—AI in healthcare spots patterns, predicts outcomes, and guides clinicians toward better decisions. And with each FDA clearance—from AI ECGs to pathology co-pilots and at-home diagnostic tools—the future of medical diagnostics feels less futuristic and more tangible.
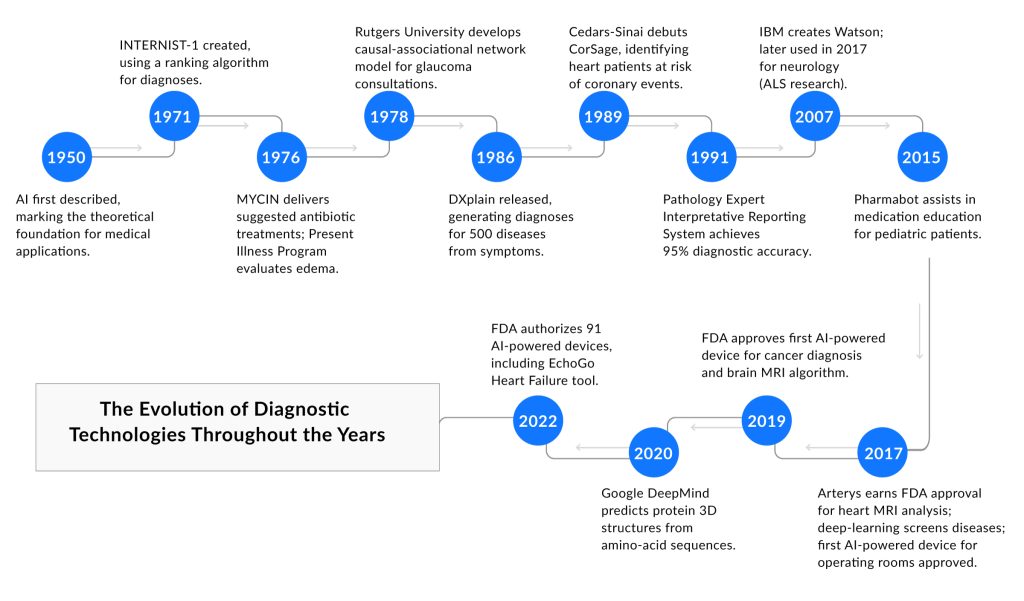
The Evolution of Diagnostic Technologies
The development of AI in medical diagnostics began in the 1950s with the introduction of artificial intelligence concepts, leading to practical applications by the 1970s. Early systems like INTERNIST-1 (1971) and MYCIN (1976) marked the start of AI diagnostics, assisting with diagnosis ranking and antibiotic recommendations. In the 1980s and 1990s, medical diagnostic AI advanced with tools like DXplain (1986), which generated diagnoses from symptoms, and CorSage (1989), which predicted coronary risks, laying the foundation for broader use.
The 2000s brought significant progress in AI in diagnostic imaging with the rise of deep learning, enabling faster and more accurate analysis. By 2017, systems like Arterys gained FDA approval for rapid heart MRI evaluations, driving growth in the artificial intelligence in medical imaging market. By 2022, the FDA had authorized 91 AI-powered devices, including EchoGo for heart failure detection, showing how AI diagnostics improved imaging tasks like X-ray and MRI analysis.
Today, AI in medical diagnostics enhances healthcare by improving diagnostic accuracy and efficiency, especially in diagnostic imaging for conditions like cancer. The artificial intelligence AI in the medical imaging market continues to expand, supported by technological advancements, though issues like data privacy remain. From its origins in the 1950s to its current role, medical diagnostic AI has become a vital tool, transforming how diseases are identified and treated.
AI-Powered Diagnostic Tools and Technologies
AI in diagnostics is no longer experimental — it’s actively deployed across hospitals, labs, and even smartphones. From analyzing scans to detecting rare mutations in DNA, AI diagnostics are reshaping how and when diseases are identified. These tools offer faster, more accurate, and scalable solutions across specialties by leveraging machine learning, neural networks, and vast datasets.
A key milestone in this evolution was the FDA’s 2018 approval of IDx-DR, the first-ever autonomous AI-powered diagnostic tool capable of detecting diabetic retinopathy without clinician input. This groundbreaking approval marked the beginning of a new era in medical diagnostic AI, where tools not only assist physicians but can independently evaluate and make diagnostic decisions in specific cases.
The current wave of AI in medical diagnostics encompasses technologies that learn patterns in massive data, whether visual, genomic, or molecular. Unlike traditional systems that rely on pre-defined rules, these tools adapt and improve continuously. As such, the future of diagnostics isn’t just digital — it’s intelligent.
AI in Medical Imaging Analysis
Artificial intelligence diagnostic imaging is one of the most mature areas of AI adoption. In radiology, deep learning models detect anomalies in X-rays, CT scans, and MRIs with sensitivity often rivaling human experts. In pathology, AI helps identify cancerous patterns in digitized tissue samples, reducing human error and expediting review.
AI in diagnostics has extended to ophthalmology, dermatology, and even cardiology. These applications not only support specialists but, in some cases, democratize care by enabling generalists or remote centers to access expert-level interpretations.
Medical diagnostic AI tools dramatically cut down the time needed to interpret imaging — from hours to seconds. Speed is critical in trauma or stroke care, where faster decisions save lives. AI also enhances accuracy by catching subtle patterns or early-stage signs that might escape the human eye.
Beyond speed and accuracy, AI diagnostics increase efficiency. Radiologists can prioritize critical cases flagged by AI, reduce burnout, and handle more patients without sacrificing quality. These tools also allow for continuous learning, improving over time with exposure to new data.
AI in Liquid Biopsy Analysis
Liquid biopsy — testing for disease-related biomarkers in blood — has gained momentum, especially in oncology. AI in diagnostics enhances this approach by processing and interpreting complex signals from circulating tumor DNA (ctDNA), exosomes, or microRNAs, identifying disease earlier than traditional methods.
This is especially critical in cancers like lung, pancreatic, or ovarian, where early detection dramatically improves outcomes. AI models can sift through minute molecular signatures in blood samples and predict disease presence or recurrence risk with high precision.
AI-powered liquid biopsy tools don’t stop at detection — they personalize the entire care path. By analyzing biomarker profiles, AI in medical diagnostics helps stratify patients into risk categories and guides therapy choices, aligning with the broader goals of precision medicine.
This is especially valuable in guiding immunotherapies or targeted treatments where efficacy depends on specific molecular alterations. By integrating data across patient populations, medical diagnostic AI tools can identify which profiles respond best to which drugs — offering hope for truly individualized care.
AI in Genomics and Genetic Testing
The fusion of genomics and AI in diagnostics is driving a new wave of precision medicine. With each genome holding billions of data points, manual interpretation is unfeasible—this is where medical diagnostic AI excels. Machine learning models can analyze sequencing data to uncover disease-linked patterns, rare mutations, and polygenic risk scores for conditions like cancer, diabetes, and cardiovascular disease.
By combining AI medical imaging with genomic insights, diagnostics are becoming more integrative, offering a comprehensive view that merges structural and molecular data, and enabling earlier, more personalized interventions.
Analyzing Genetic Data for Disease Risk and Diagnosis
Algorithms trained on large genomic datasets can detect mutations associated with inherited and complex diseases. From identifying BRCA mutations in cancer to predicting risks for neurodegenerative or metabolic disorders, AI diagnostics offer predictive insights at scale. This is especially pivotal in the realm of Artificial Intelligence in cancer diagnostics, which, according to DelveInsight, was valued at USD 1 billion in 2024 and is projected to grow at a CAGR of 11.16% from 2025 to 2032, reaching USD 2.4 billion by 2032—underscoring how early detection and personalized treatment planning can significantly improve survival rates.
Beyond single-gene disorders, AI maps interactions between thousands of genes and environmental inputs—advancing our understanding of multifactorial diseases and supporting early, customized healthcare strategies. As AI continues to evolve, its integration into genomics is not only refining risk prediction but also reshaping the future of precision medicine.
While we take the plunge into the deeper dimensions of AI, don’t miss our dedicated blog on Artificial Intelligence in Cancer Diagnostics—a focused exploration of how AI is transforming oncology through earlier detection, smarter treatment decisions, and improved patient outcomes.
AI for Drug Response Prediction
In pharmacogenomics, medical diagnostic AI is revolutionizing treatment planning by transforming how clinicians approach prescribing. By analyzing gene-drug interactions, AI in diagnostics predicts how a patient will metabolize medications, anticipates potential side effects, and identifies the likelihood of therapeutic success, minimizing the traditional trial-and-error approach. In oncology, AI diagnostics can forecast tumor resistance patterns, enabling oncologists to select the most effective therapies early on. In psychiatry, it aids in tailoring antidepressant regimens based on individual genetic markers, leading to improved patient outcomes.
These advancements align closely with the broader role of Artificial Intelligence in drug commercialization, where AI is driving faster, more precise decision-making from R&D to patient care. Reflecting this momentum, the artificial intelligence in the drug commercialization market is projected to grow at a robust CAGR of 24.12% from 2025 to 2032. Together, these innovations are reshaping healthcare into a more proactive, personalized, and data-driven system.
AI in Point-of-Care Diagnostics
Point-of-care diagnostics are transforming healthcare by bringing testing closer to the patient, eliminating delays typically associated with centralized lab testing. When combined with AI in medical diagnostics, these tools become even more powerful. AI algorithms can rapidly analyze biosignals, medical images, and patient data from portable devices, enabling faster and more reliable diagnoses right at the bedside or in remote locations. This shift is not only improving clinical efficiency but also ensuring timely care in critical and underserved settings.
AI-Powered Devices for Rapid Diagnosis
Point-of-care diagnostic devices — from portable ultrasound to AI-enhanced handheld ECG monitors — bring lab-grade diagnostics to the bedside. AI in diagnostic imaging within these tools allows frontline clinicians to get immediate, decision-ready results.
Take AI-integrated stethoscopes that detect heart murmurs or smart thermometers analyzing symptoms for possible infections. These innovations reduce the delay between testing and treatment, especially critical in urgent care or rural settings.
Improving Accessibility to Diagnostics
AI diagnostics extend access to healthcare where it’s needed most. In underserved or resource-limited areas, AI-powered mobile apps and devices can bridge gaps in specialist availability, offering diagnostic capabilities once restricted to tertiary care hospitals.
These tools are empowering community health workers, enabling early triage, and reducing unnecessary referrals. Combined with cloud connectivity, they also support centralized review and population-level disease monitoring, boosting global health equity.
The Role of AI in Modern Diagnostics
The integration of AI in diagnostics marks a pivotal shift in healthcare—moving from reactive to predictive and personalized care. Unlike traditional methods that often rely on limited human interpretation and static criteria, medical diagnostic AI leverages machine learning and deep neural networks to process vast volumes of clinical data, medical images, and patient histories in real time.
Whether it’s AI in diagnostic imaging that detects abnormalities invisible to the naked eye or genomic AI tools that predict disease risk based on genetic variants, these innovations bring precision to every level of diagnosis. With continuous learning, these AI models evolve with each new data point, becoming more accurate and insightful over time.
The role of AI diagnostics is no longer confined to research—it is already influencing clinical workflows across radiology, pathology, cardiology, and primary care. From speeding up diagnosis to eliminating human error, AI is now seen as an essential co-pilot for clinicians.
How AI Is Transforming Healthcare Diagnostics with Unprecedented Accuracy
Diagnostic accuracy is the cornerstone of effective treatment, and this is where AI in medical diagnostics shines. Studies show that AI-powered diagnostic tools can outperform even experienced clinicians in tasks like identifying malignant tumors, classifying skin lesions, or flagging early signs of diabetic retinopathy.
What gives AI in diagnostics its edge is its ability to process and learn from multimodal data—imaging, lab results, genomics, and EHRs—to deliver more holistic and precise conclusions. For instance, in AI in diagnostic imaging, convolutional neural networks (CNNs) can detect microcalcifications in mammograms with greater consistency than radiologists, reducing both false positives and missed cases.
Furthermore, AI reduces diagnostic variability. While human interpretation can vary by skill level and fatigue, AI diagnostics deliver consistent performance, ensuring uniform care standards across institutions and geographies.
Case Studies: Successful AI Implementations in Diagnostics
AI’s potential isn’t just theoretical—it’s proven through real-world success stories. Take PMcardio-STEMI, a recently FDA-designated AI-powered ECG model by Powerful Medical, which detects STEMI heart attacks with cardiologist-level precision using just a smartphone and ECG input. This is a breakthrough in medical diagnostic AI, especially in emergency settings where time is critical.
In the realm of AI in diagnostic imaging, Subtle Medical’s SubtleMR—cleared by the FDA—enhances the quality of MRI scans using AI, allowing for faster imaging without compromising accuracy. It’s now in use across hospitals to increase throughput and improve diagnostic confidence.
Another compelling case is Cytovale’s IntelliSep, an FDA-cleared AI diagnostic tool that rapidly detects sepsis risk within minutes of patient triage. By analyzing immune system activation patterns through machine learning, it significantly reduces diagnostic time compared to traditional methods.
These implementations highlight the evolving landscape of AI in medical diagnostics, where the fusion of data science and clinical expertise is delivering safer, faster, and smarter healthcare.
Latest Developments in AI-Powered Diagnostic Devices
The field of AI-powered diagnostic devices has seen remarkable advancements, with several innovations receiving regulatory approvals and designations. Here are some of the latest developments:
April 2025
Quite recently, on April 8th, Proprio marked a groundbreaking moment in AI diagnostics with the FDA’s second 510(k) clearance for its Paradigm platform—the world’s first AI surgical guidance platform to enable real-time intraoperative measurements. This milestone elevates artificial intelligence from a passive observer to an active participant in surgical precision, ushering in a new era for AI in healthcare diagnostics.
For the first time, surgeons can dynamically visualize 3D anatomy, track segmental alignment, and measure progress against pre-operative plans—all without halting procedures or exposing teams to radiation. Paradigm’s AI-driven diagnostic tools bring cognitive clarity to the OR, reducing revision surgeries, accelerating recovery, and improving outcomes for patients worldwide.
Adopted by top-tier institutions like Duke Health and UW Medicine, and trained on a growing trove of surgical data curated by elite surgeons, Proprio’s technology is not just transforming individual surgeries—it’s redefining the foundation of surgical diagnostics. This breakthrough is a bold step forward in the future of AI medical imaging and real-time, intelligent decision-making in healthcare.
Earlier in the month, Paige earned the FDA Breakthrough Device designation for PanCancer Detect, an AI-powered diagnostic tool that assists pathologists in identifying cancer across multiple tissues and organs. It’s the first AI tool of its kind designed to detect both common and rare cancers from various anatomic sites.
With rising demand and a shortage of pathologists, PanCancer Detect helps streamline workflows, flag critical cases, and accelerate diagnosis. This recognition adds to Paige’s growing list of regulatory achievements, including FDA authorization for Paige Prostate Detect and designations for tools like Paige Lymph Node.
Integrated within the Paige Alba™ platform, this latest innovation reflects the company’s commitment to advancing precision medicine and transforming cancer diagnostics through cutting-edge AI.
March 2025
In March 2025, Accelerate Diagnostics submitted its Accelerate WAVE™ system and gram-negative test kit to the FDA for 510(k) clearance. Designed to deliver rapid antimicrobial susceptibility testing (AST) from blood cultures in just 4.5 hours, the WAVE system aims to enable same-shift, targeted treatment for serious infections like sepsis. With its scalable design and high-throughput capability, this AI-powered diagnostic tool supports faster clinical decisions, better patient outcomes, and a stronger defense against antimicrobial resistance—a major global health threat.
Also in the same month, Caristo Diagnostics received FDA 510(k) clearance for CaRi-Plaque, an AI-assisted diagnostic tool that analyzes coronary CT angiography (CCTA) scans to detect and assess coronary plaque and artery narrowing. Built on the proven CaRi-Heart platform, this AI-powered diagnostic technology helps identify both plaque buildup and the hidden inflammation that often triggers sudden heart attacks—offering clinicians a proactive approach to coronary artery disease. With seamless cloud-based workflows and CPT code coverage coming in 2026, CaRi-Plaque marks a significant leap forward in AI cardiovascular diagnostics, enabling earlier, personalized intervention and potentially saving countless lives.
Earlier that month, Powerful Medical’s PMcardio STEMI AI ECG model received FDA Breakthrough Device Designation for its unique ability to detect both STEMI and STEMI equivalents—life-threatening heart attacks often missed by standard ECGs. As the only AI diagnostic tool of its kind, PMcardio enables faster, more accurate triage, especially in settings without immediate specialist access. This designation not only fast-tracks its market approval but also highlights the growing impact of AI in diagnostics, particularly in improving emergency cardiac care and saving lives through earlier, precision-driven interventions.
In February, Ibex Medical Analytics secured FDA 510(k) clearance for Ibex Prostate Detect, an AI-powered diagnostic tool designed to support pathologists in identifying missed or subtle prostate cancers from scanned biopsy images. This software-only device analyzes whole slide images of H&E-stained prostate core needle biopsies and flags suspicious areas using visual heatmaps, enhancing diagnostic accuracy. In clinical studies, it demonstrated a 99.6% positive predictive value and revealed a 13% rate of missed cancers in initially benign diagnoses—cases later confirmed by expert pathologists. With this clearance, Ibex Prostate Detect strengthens the role of AI in cancer diagnostics and reinforces digital pathology’s readiness for broader clinical use.
February 2025
Earlier in February, Huxley Medical received FDA clearance for its SANSA home sleep apnea test to use cellular data uploads, eliminating the need for Bluetooth pairing or apps, streamlining the sleep apnea diagnostic process, and improving test completion rates. SANSA is the only hands-free, wire-free, app-free test, combining nine physiological channels, including ECG, into a single chest patch for accurate, AI-supported insights into cardiopulmonary health.
In the same month, RapidAI gained FDA 510(k) clearance for Lumina 3D, the first automated 3D imaging reconstruction solution. Integrated into the Rapid Enterprise Platform and deployed on Rapid Edge Cloud, Lumina 3D enables real-time, mobile-accessible visualization of neurovascular anatomy, enhancing workflow efficiency, catheter planning, and diagnostic accuracy while reducing procedure times and supporting overburdened radiology teams.
January 2025
January 2025 was more than just the start of a new year — it was a defining moment for AI diagnostics. As the world leaned harder on technology to ease clinical workloads and deliver faster, more accurate decisions, innovation answered. The tone was set early with Modella AI’s PathChat DX — a generative AI-powered digital co-pilot for pathologists — receiving the FDA’s Breakthrough Device Designation. Built on a multimodal foundation model trained on histology and clinical data, it offers real-time case interpretation and structured reporting, hinting at a future where AI doesn’t replace specialists but amplifies them.
This wave of smarter diagnostics extended to emergency medicine. Cytovale’s IntelliSep test, which reads immune responses to detect sepsis risk in under 10 minutes, marked one year since FDA clearance — and it’s gaining serious ground. Backed by $100 million in Series D funding, Cytovale is rapidly scaling deployment in U.S. emergency departments. The drive to accelerate sepsis triage mirrors the same urgency that fuels AI development in pathology: timely, automated insights that clinicians can act on without delay.
The need for speed was equally urgent in sexual health diagnostics. This January, Roche’s cobas liat system was cleared and granted a CLIA waiver for new multiplex assays targeting common STIs. With results available in just 20 minutes, even in non-lab settings, it brings decentralized precision to an area long plagued by delayed interventions. Just like IntelliSep and PathChat DX, it reflects a growing theme: diagnostics are moving closer to the point of care — faster, more accessible, and more aligned with real-world clinical workflows.
Meanwhile, AI cancer diagnostics took a precision leap forward. Tempus AI launched xT CDx, a 648-gene NGS panel newly cleared by the FDA. Capable of detecting tumor mutations, microsatellite instability, and more, the test supports both therapy selection and clinical trial enrollment, streamlining personalized treatment pathways. As with pathology and sepsis, oncology is increasingly powered by layered data and AI integration — bringing clarity to complexity at scale.
Even MRI, a domain often untouched by digital transformation, saw a patient-centric shift. InkSpace Imaging received FDA clearance for its 1.5T, 24-channel MR coil, designed to work with Siemens scanners. Lightweight, flexible, and easy to set up, the coil offers a smoother experience for both patients and technologists while maintaining image quality.
Rounding out the month, Roche returned with another milestone when the FDA expanded clearance of its Roche Digital Pathology Dx suite to include the high-capacity VENTANA DP 600 slide scanner. Integrating seamlessly with the already cleared DP 200 and Roche’s digital workflow software, this update offers pathology labs enhanced throughput and resolution for cancer diagnostics. Coming on the heels of PathChat DX’s AI advancement, the timing couldn’t be more serendipitous, reinforcing the narrative that digital transformation in pathology is no longer optional, but essential.
Together, these six milestones turned January 2025 into a watershed moment for diagnostics. From generative AI to high-speed imaging, from rapid infectious disease testing to comprehensive tumor profiling, the month made one thing clear: the future of diagnostics is faster, smarter, and more patient-centered than ever before.
Key Companies in the AI Diagnostics Market
The field of AI in diagnostics is being transformed by a powerful combination of deep learning, imaging innovation, and real-time data interpretation. Pioneers like iCAD, Inc., ibex-ai, PathAI, Kheiron Medical Technologies, Paige AI, MVision AI, and Digital Diagnostics Inc. are redefining how clinicians detect and interpret diseases—from cancer to chronic conditions—using AI-first platforms. At the same time, major tech and healthcare leaders such as NVIDIA Corporation, Siemens Healthineers AG, GE HealthCare, and Roche Diagnostics are playing a key role in scaling medical diagnostic AI solutions across global markets. Their efforts, spanning radiology, pathology, and genomics, are fueling rapid growth in the AI diagnostics market, driven by multimodal AI and powerful diagnostic engines that are reshaping both clinical workflows and commercial landscapes.

Adding to this innovation wave are companies working across specialized diagnostic domains. Flatiron Health, Freenome Holdings, ConcertAI, and Onc.AI are pushing boundaries in oncology AI and real-world evidence generation. Meanwhile, firms like Abbott, Bio-Rad Laboratories, Agilent Technologies, BD, and QIAGEN are embedding AI into molecular diagnostics, infectious disease screening, and biomarker analysis. Even emerging players like Azra AI, Sonrai Analytics, and Therapixel are making notable strides in algorithmic precision. Together, these companies are advancing AI diagnostics into mainstream clinical practice—delivering faster, smarter, and more personalized care across the globe.
Regulatory Landscape for AI in Diagnostics
As AI diagnostics move from concept to clinical implementation, regulatory bodies are evolving to keep pace. The FDA has taken significant steps to regulate AI/ML-based Software as a Medical Device (SaMD), ensuring both safety and innovation.
In April 2019, the FDA released a discussion paper proposing a total product lifecycle (TPLC) regulatory approach tailored to AI/ML. Unlike traditional software, AI systems often learn and evolve after deployment, requiring a flexible yet robust oversight framework.
Key FDA initiatives include:
- Predetermined Change Control Plan (PCCP): Allows manufacturers to outline expected algorithm updates and how they’ll manage safety post-deployment.
- Good Machine Learning Practices (GMLP): Encourages developers to build transparent, reproducible, and high-quality AI systems.
- Digital Health Software Precertification Program: Aims to streamline approval for companies with a strong culture of quality and real-world performance monitoring.
While a few AI-powered diagnostic devices—like IDx-DR for diabetic retinopathy—have gained FDA clearance, the path to widespread regulatory acceptance remains under construction.
Impact of AI Diagnostics on Patient Care
The transformative power of medical diagnostic AI lies in its ability to improve clinical decision-making, reduce diagnostic errors, and ultimately enhance patient care.
Taking the example of stroke detection—AI-powered tools like Viz.ai can analyze CT angiograms and alert neurovascular teams within minutes, drastically reducing the time to intervention. In cancer detection, AI systems are capable of analyzing digital pathology slides at scale, flagging potentially malignant patterns faster and more accurately than manual inspection.
Moreover:
- AI accelerates diagnosis, especially in time-sensitive conditions like sepsis or cardiac arrest.
- It enhances consistency, reducing inter-observer variability.
- It supports clinicians with real-time data synthesis and predictive insights.
In underserved areas, AI tools deployed on mobile or cloud-based platforms bring sophisticated diagnostics closer to the point of care, bridging healthcare gaps.
Emerging Trends, Opportunities, and Challenges in AI Diagnostics
AI in diagnostics is rapidly evolving from a supportive tool to an integral part of clinical workflows. Innovations like multimodal diagnostics, which fuse radiology, genomics, pathology, and clinical notes, are pushing boundaries toward more holistic patient assessments. Technologies such as federated learning and explainable AI (XAI) are tackling core issues of data privacy and interpretability, while AI-powered wearables bring real-time, remote diagnostics to the forefront.
These advancements open the door to earlier disease detection and intervention, allowing clinicians to identify conditions at a stage when they are most treatable. With the help of predictive analytics, AI enables personalized treatment strategies tailored to individual patient profiles, improving outcomes and reducing trial-and-error in therapy selection.
Additionally, AI brings greater efficiency to healthcare delivery by automating routine tasks, streamlining workflows, and freeing up valuable time for clinicians to focus on direct patient care.
However, challenges remain. Integration with outdated EHR systems, the lack of development standards, and legal ambiguities around AI accountability continue to hinder widespread adoption.
To truly realize the promise of AI in medical diagnostics, seamless collaboration among developers, clinicians, and regulators is essential—balancing innovation with trust, safety, and equity.
Conclusion
The future of diagnostics is no longer on the horizon—it’s already here, shaped by algorithms, accelerated by data, and driven by the pursuit of better care. AI in diagnostics is not just a tool; it’s becoming the heartbeat of next-gen medicine.
From interpreting images with superhuman accuracy to detecting diseases before symptoms appear, AI in medical diagnostics is unlocking a new era—one where every pixel, gene, and heartbeat can tell a story. And with AI diagnostics embedded into clinical workflows, doctors are not being replaced—they’re being empowered.
Of course, every revolution needs guardrails. As medical diagnostic AI continues to evolve, the need for responsible innovation, transparent algorithms, and trust-building mechanisms becomes ever more vital. The challenge is not just in building smarter systems—but in ensuring those systems are safe, ethical, and inclusive.
The journey is just beginning, and the roadmap is being drawn in real-time. As we look ahead, the momentum behind AI diagnostics is undeniable. The focus now is on scaling these innovations responsibly—bridging the gap between possibility and real-world impact.
Downloads
Article in PDF
Recent Articles
- How is Artificial Intelligence (AI) Playing a Constructive Role in Mental Health Management?
- Noxxon’s NOX-A12 clinical trial; Exact Sciences buys PreventionGenetics; Nuvalent’s clinical trai...
- Neuromodulation Devices Observed to Reshape the Migraine Treatment Dynamics
- A promising immune-stimulating target
- Norlase’s ECHO™ Green Pattern Laser; HAPPE Spine’sINTEGRATE-C™ Interbody Fusion Sys...



